Texas Insight provides timely reporting of healthcare issues and events as they occur within the legislative and regulatory branches of Texas state government.

HHSC: Pediatric Acute-Onset Neuropsychiatric Syndrome Advisory Committee. The Council gathered to discuss Agency and program overviews; discuss the role and function of the Pediatric Acute-Onset Neuropsychiatric Syndrome Advisory Council; hear an Advisory Committee Coordination Office overview, an Open Meetings Act overview, and an Ethics overview; and take public comment. Read the report.
HHSC: Texas Council on Cardiovascular Disease and Stroke. The Council gathered to plan for representation and guidelines for 87th Legislative Session; discuss Chronic Care Workgroup and Stroke Survivors and Caregivers Conference Report; discuss Agency Representative Reports and Liaison Reports; and take public comment. Read the report.
HHSC: Nursing Facility Payment Methodology Advisory Committee (NF-PMAC). The Committee gathered to discuss subcommittee intent; discuss calculation of the base rate and case-mix index for direct care and other resident care; hear a presentation on Texas-specific case mix; discuss maintenance of the resource utilization group with reduced levels; and take public comment. Read the report.
HHSC: Medical Care Advisory Committee. The Committee gathered to discuss the Medicaid and Children’s Health Insurance Program (CHIP) activities; discuss Texas managed care quality strategy; vote on action items, and hear informational items. Read the report.
HHSC: Value-Based Payment and Quality Improvement Advisory Committee. The Committee gathered to hear an update on the new member appointment process; discuss the 2020 Legislative Report; hear a staff update on Medicaid value-based activities; discuss plans for 2021; and take public comment. Read the report.
HHSC: Texas HIV Medication Advisory Committee. The Committee gathered to hear Commissioner’s Updates, a THMP update, and subcommittee reports; and take public comment. Read the report.

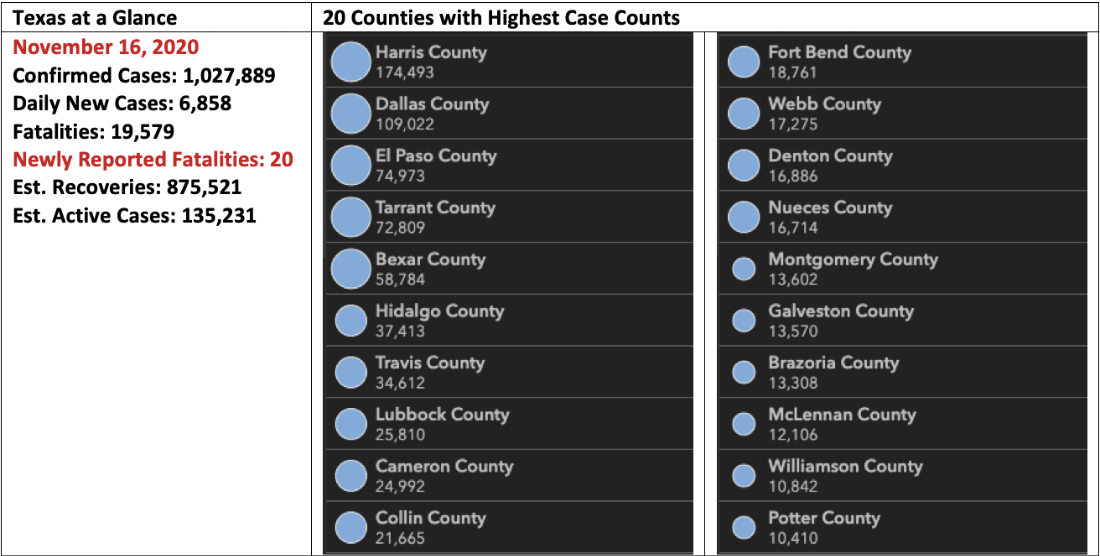
November 17, 2020
- This meeting will be webcast: Perinatal Advisory Council (PAC) Agenda
- This meeting will be webcast: Notice of Public Hearing on Proposed Amendment to §355.8201, relating to Waiver Payments to Hospitals for Uncompensated Care.
November 18, 2020
- This meeting will be webcast: State Preventive Health Advisory Committee (SPH) Fiscal Year 2021- Meeting #1 Agenda
- State Medicaid Managed Care Advisory Committee Administrative Simplification Subcommittee Agenda
November 19, 2020
- This meeting will be webcast: Health and Human Services Commission Executive Council (HHSC EC) Agenda
- This meeting will be webcast: State Medicaid Managed Care Advisory Committee (SMMCAC) Agenda
November 20, 2020
- This meeting will be webcast: Sickle Cell Task Force (SCTF) Agenda

Texas
- As Texas universities prepare to send thousands of students home for the holidays, few are requiring COVID-19 safety precautions
- Texas leaders stick with COVID-19 reopening plan as other states lock down
- Texas reports more than 1 million COVID-19 cases, but state officials are slow to act
- Incarcerated Texans enlisted to work in county morgue as COVID-19 deaths overwhelm El Paso
- Austin Public Health creates coronavirus vaccine distribution plan as trials show promise
U.S.
- ‘How Did We Not Know?’ Gun Owners Confront a Suicide Epidemic
- CDC quietly removes guidance pushing for school reopenings
- Pandemic Delivers a Triple Whammy to Working Women
- Deep Sleep Protects Against Alzheimer’s, Growing Evidence Shows
- Former COVID-19 crisis planner: Don’t expect ‘military miracle’ on vaccine distribution
- Immunity to the Coronavirus May Last Years, New Data Hint
- Why Does Pfizer’s COVID-19 Vaccine Need To Be Kept Colder Than Antarctica?
- Study finds some mouthwashes can kill coronavirus
- Vaccine Optimism
- ‘We Are Shipping To The U.S.’: Inside China’s Online Synthetic Drug Networks
- Leading hospital, doctor associations call on Trump to share COVID-19 information with Biden
- Biden’s Economic Plan for the Virus
- Amazon Wants To Sell You Prescription Medications
- Poll shows growing awareness of high-risk COVID-19 activities among Republicans
- After holding out, Republican governors reverse course and issue mask mandates.
- Experts: Gobble All You Like, But Do It With The Turkeys In Your Own Household
- Pfizer launching pilot vaccine delivery program in four states
- ‘More People May Die’ Because of Trump’s Transition Delay, Biden Says



No COVID-19 Webinars with LTCR for NF or ALF Providers Next Week. LTCR will not be conducting COVID-19 webinars the week of Thanksgiving. Providers can join us for the COVID-19 webinars the following week.
The next NF webinar will be held on December 2 at 2:30 p.m.
The next ALF webinar will be held on December 3 at 11 a.m.

HHSC Publishes Updated COVID-19 Frequently Asked Questions for ALF Providers. HHSC has published updated COVID-19 Frequently Asked Questions (PDF) for assisted living facility providers.

New State Reporting Requirements for Facilities Currently Reporting COVID-19 Lab Data to the National Healthcare Safety Network. Effective November 14, 2020, long-term care facilities who are currently submitting COVID-19 laboratory data into NHSN should discontinue their direct reporting to the Department of State Health Services’ National Electronic Disease Surveillance System.
Reporting through NHSN will fulfill the state reporting requirement for facilities actively entering into NHSN. It is important to note that facilities must continue to comply with their local health authority directive for reporting.
Any long-term care facilities who are NOT reporting to NHSN must continue to report to DSHS NEDSS.
Thank you for your cooperation. Questions may be emailed to COVID-19ELR@dshs.texas.gov.

Food and Drug Administration. The U.S. Food and Drug Administration announced the following:
- The FDA updated its guidance on investigational COVID-19 convalescent plasma.
- The agency published a new webpage, A Closer Look at COVID-19 Diagnostic Testing, to provide health care providers and other public health professionals, including those who might purchase COVID-19 tests, more technical information and resources.
- Testing updates:
- As of today, 288 tests are authorized by the FDA under EUAs; these include 223 molecular tests, 58 antibody tests, and 7 antigen tests.

National Institutes of Health. Promising Interim Results from Clinical Trial of NIH-Moderna COVID-19 Vaccine. An independent data and safety monitoring board (DSMB) overseeing the Phase 3 trial of the investigational COVID-19 vaccine known as mRNA-1273 reviewed trial data and shared its interim analysis with the trial oversight group on Nov. 15, 2020. This interim review of the data suggests that the vaccine is safe and effective at preventing symptomatic COVID-19 in adults. Read the full release and learn more.

Centers for Medicare & Medicaid Services. Download this document to access the following update from CMS:
- CMS Releases Nursing Home COVID-19 Training Data with Urgent Call to Action.
- UPDATED: November 13, 2020. CMS Takes Steps to Ensure Medicare Beneficiaries Have Wide Access to COVID-19 Antibody Treatment.
- Don’t Wait, Apply Now! 2020 Extreme and Uncontrollable Circumstances Exception and Promoting Interoperability Hardship Exception Applications are Due December 31
- COVID-19 Vaccine Codes and PC-ACE Software Update.
- Reminder: CMS Issues Interim Final Rule with Comment Period to Ensure Coverage of COVID-19 Vaccines & Therapeutics.
