Upcoming Public Meetings and Events. From HHSC: While every effort has been made to offer an accurate and current listing of meeting agendas and events on this calendar, the information has been compiled from a variety of sources and is subject to change without notice to the user.
Please Note: This is a comprehensive list of upcoming meetings provided by HHSC. Not every meeting fits the parameters for coverage by Texas Insight. Refer to Texas Insight’s “Weekly Insight” newsletters for regular updates on planned coverage.
For details on the postings follow this link Communications & Events | Texas Health and Human Services
***Designates meetings to be covered by Texas Insight.
April 11, 2022
9:00 am Public Hearing
April 14, 2022
9:00 am Advisory Committee
1:00 pm Advisory Committee
1:00 pm Advisory Committee
April 20, 2022
10:00 am Advisory Committee
April 22, 2022
9:00 am Advisory Committee
April 28, 2022
9:30 am Advisory Committee
HHSC has the Following Rules Available for Comment. The Administrative Procedure Act (Texas Government Code, Chapter 2001(link is external)) requires the notice published in the Texas Register to include a brief explanation of the proposed rule and a request for comments from any interested person. The notice also includes instructions for submitting comments regarding the rule to the agency, including the date by which comments must be submitted. Agencies must give interested persons “a reasonable opportunity” to submit comments. The public comment period begins on the day after the notice of a proposed rule is published in the Texas Register and lasts for a minimum of 30 calendar days.
The Administrative Procedure Act (Texas Government Code, Chapter 2001(link is external)) requires the notice published in the Texas Register to include a brief explanation of the proposed rule and a request for comments from any interested person. The notice also includes instructions for submitting comments regarding the rule to the agency, including the date by which comments must be submitted. Agencies must give interested persons “a reasonable opportunity” to submit comments. The public comment period begins on the day after the notice of a proposed rule is published in the Texas Register and lasts for a minimum of 30 calendar days. For draft rules detail please see Comment on Proposed & Draft Rules | Texas Health and Human Services or follow the links to each individual rule.
| Title | Project No. / Description | Comment End Date |
| Title 26, Chapter 556, Sections 556.2, 556.3, and 556.5 – 556.13, concerning Nu… | #21R164: NATCEP Clinical Site Expansion | 5/9/22 |
| Title 26, Chapter 745, Sections 745.609, 745.611, 745.613, 745.615, and 745.617… | #21R167: Fingerprint Criminal History Checks | 5/9/22 |
| Title 26, Chapter 742, Section 742.508, and Chapter 747, Section 747.2318, conc…
|
#21R160: Tummy Time Supervision in Child-Care Homes | 4/18/22 |
| Title 40, Chapter 30, Medicaid Hospice Program repeal
|
#20R126: Medicaid Hospice Program | 4/18/22 |
| Title 26, Chapter 266, Medicaid Hospice Program
|
#20R126: Medicaid Hospice Program | 4/18/22 |
| Title 25, Chapter 157, Subchapter C, Section 157.41, Automated External Defibri…
|
#21R142: Automated External Defibrillators for Public Access Defibrillation | 4/11/22 |
Informal Comments
Informal opportunities to comment occur before a rule is published in the Texas Register. HHS staff may solicit informal public and stakeholder input by:
- inviting stakeholders to submit comments on potential rule changes during rule development.
- sharing a draft rule with stakeholders for review.
- using existing HHS advisory committees to comment on rules.
The following are draft rules on which HHS is accepting informal public or stakeholder input. All rules are posted in MS Word format unless otherwise noted.
For detail on the rate proposals and older rate proposals please follow the link: Rate Packets | Provider Finance Department (texas.gov) or follow the live link by each proposal.
| Title | Proposed Effective Date | Documents |
| Notice of Proposed Adjustments to Fees, Rates or Charges for Medical Policy Review of Physician Administered Drugs: Vaccines & Toxoids | September 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for Mobility Aids | June 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for Collaborative Care Management Services | June 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates, or Charges for Medicaid Biennial Calendar Fee Review of the following: Long Acting Reversible Contraceptives (LARCs) | April 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates, or Charges for Policy Fee Review of the following: Prosigna | April 01, 2022 |
|
| Notice of Proposed Temporary Rate Actions for American Rescue Plan Act (ARPA) Home and Community-Based Services (HCBS) Provider Retention Payments | March 01, 2022 | · Notice of Proposed Temporary Rate Actions for American Rescue Plan Act (ARPA) Home and Community-Based Services (HCBS) Provider Retention Payments |
| Biennial Calendar Fee Review: Vaccines and Toxoids Q Codes NDCX | March 01, 2022 |
03-01-2022-biennialcalendarfeereview-vaccine-toxoids-qcodes-ndcx.pdf |
| Notice of Proposed Medicaid Payment Rates for the Medical Policy Review of End Stage Renal Dialysis Revenue Codes | March 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for IV Therapy Equipment and Supplies | March 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for Nutritional (Enteral) Products, Supplies, and Equipment – Home Health & CCP: Immobilized Lipase Cartridge | March 01, 2022 |
|
| Proposed Rate Actions for Fiscal Year 2022-23 Quarter 1 Biennial Fee Review | March 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges Healthcare Common Procedure Coding System (HCPCS) | March 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for Cardiac Magnetic Resonance Imaging | March 01, 2022 |
|
| Biennial Calendar Fee Review | March 01, 2022 |
03-01-2022-biennialcalendarfeereview.pdf
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for Q Codes | March 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates, or Charges for Medicaid Policy Review of Autism Services | February 01, 2022 |
|
Attorney General
No Report This week
Office of the Governor
Governor Abbott Appoints Seven To Advisory Council On Emergency Medical Services. Governor Greg Abbott has appointed Peter Marocco to the Advisory Council on Emergency Medical Services, known as GETAC, for a term set to expire on January 1, 2026. Additionally, the Governor has appointed Billy “Scott” Lail, Daniel “Danny” Ramirez and Katherine “Kate” Remick, M.D. and reappointed Jeffery “Jeff” Barnhart, Ruben A. Martinez and Ryan Matthews for terms set to expire on January 1, 2028. The council recommends changes to the EMS/Trauma System to ensure that communities receive comprehensive and efficient emergency care.
Peter Marocco of Dallas is an investor and the Managing Director of Endstate Strategies LLC. He is a Club for Growth Foundation fellow and serves on the advisory board for the Foreign Policy Research Institute. Marocco received a Bachelor of Science in International Affairs from Florida State University and Master of International Human Rights Law from the University of Oxford.
Billy “Scott” Lail of Glen Rose is the Fire Chief for the City of Cleburne Fire Department. He is a member of the International Association of Fire Chiefs and the Texas Fire Chief’s Association and a former member of the International Association of Firefighters. He volunteers as the Deacon at Stephenville Christian Reformed Church. Lail received an associate’s degree in Fire Science from Cleburne Hill College.
Daniel “Danny” Ramirez of San Juan is the EMS Chief for the City of Pharr. He is a member of the Texas City Managers Association and the Texas EMS Association. He also volunteers for the Pharr Volunteer Fire Department. Ramirez received a degree as an Emergency Medical Technician from Texas State Technical College.
Katherine “Kate” Remick, M.D. of Austin is an associate professor for Dell Medical School at The University of Texas at Austin, and serves as the Medical Director for San Marcos Hays County EMS System. She is a member of the American College of Emergency Physicians, National Association of EMS Physicians, American Academy of Pediatrics, and the National Prehospital Pediatric Readiness Steering Committee. Additionally, she is the Medical Director for the Emergency Pediatric Care Course with the National Association of Emergency Medical Technicians, volunteer for the Research Leadership Group for ESO Solutions, and co-director for the National Pediatric Readiness Project. Remick received a Bachelor of Science in Physics and Molecular and Cellular Biology from the University of Arizona and a Doctor of Medicine from The University of Texas Medical Branch.
Jeffery “Jeff” Barnhart of Canyon is the CEO of the Deaf Smith County Hospital District in Hereford and serves as an adjunct professor at the Texas Tech University Health Sciences Center. He is a member of the American College of Healthcare Executives and the Emergency Medical Task Force-1. Additionally, he is a member of Hereford Lions Club and volunteer board member of the Turn Center. Barnhart received a Bachelor of Arts in Healthcare Management from Ottawa University and a Master of Science in Clinical Practice Management from the Texas Tech University Health Sciences Center.
Ruben A. Martinez of Concepcion is President and CEO of Zenitram Enterprises LLC. He served on the Youth Advisory Board for the National Institute of Health and the COVID Prevention Network. He is the former President of the Chancellors Student Advisory Council for the Texas A&M System and former Student Body President at Texas A&M University–Kingsville. Martinez received a Bachelor of Arts in Political Science from Texas A&M University–Kingsville.
Ryan Matthews of Holliday is the President and CEO of Trans Star Ambulance in Wichita Falls and is a licensed paramedic. He is a member of the Texas EMS Alliance, American Ambulance Association, and the Texas State Firemen and Fire Marshal’s Association. Additionally, he is a volunteer fireman and first responder for Holliday Fire and Rescue and serves as a North Texas representative for the Emergency Medical Task Force. Matthews received a Bachelor of Science in Biology and Chemistry from Texas Tech University.
Department of Family and Protective Services
No Report This week
Department of State Health Services
DSHS Coronavirus Disease 2019 (COVID-19) Update
Updated Information for COVID-19 Vaccination Providers:
- COVID-19 Vaccine Provider Webinars (last updated 4/5/22)
Main COVID-19 Site Updates:
- Information for COVID-19 Therapeutics Providers (last updated 4/8/22)
- News & Updates
- ADDED: Sotrovimab No Longer Authorized as of 4/5/22
Sotrovimab is no longer authorized for use in Texas, effective immediately. See the 4/5/22 DSHS letter to therapeutics providers for complete details. - Weekly eDigests (PDFs)
- ADDED: Sotrovimab No Longer Authorized as of 4/5/22
- Provider Resources & Training
- Job Aids & FAQs
- UPDATED: Therapeutics Product Guide – Job Aid (PDF, updated 4/08/2022)
- UPDATED: Health Partner Ordering Portal (HPOP) FAQs (PDF, V.2.0, updated 4/05/2022)
- Job Aids & FAQs
- News & Updates
- COVID-19 Therapeutics Information (last updated 4/8/22)
- ADDED: What Is the Test to Treat Program?
- ADDED: Test to Treat Site Locator
- ADDED: What Is the Test to Treat Program?
If you have any questions or would like more information about the content on this website, please contact coronavirus@dshs.texas.gov.
DSHS Flu Surveillance Activity Report Update.
This information has recently been updated and is now available. Compared to the previous week, the percentage of specimens testing positive for influenza reported by hospital laboratories has decreased. The percentage of patient visits due to influenza-like illness (ILI) has decreased. No influenza-associated pediatric deaths were reported. No influenza-associated institutional outbreaks or school closures were reported.
DSHS RSV Data Update. This information has recently been updated and is now available. RSV surveillance in Texas is based on data collected through the National Respiratory and Enteric Virus Surveillance System ( NREVSS) sponsored by the Centers for Disease Control and Prevention (CDC). This is a laboratory-based sentinel surveillance system and participation is voluntary. Sentinel providers report both the number of RSV tests performed and the number of positive tests detected on a weekly basis. Testing is typically by antigen detection; however, viral isolation (i.e., culture) and polymerase chain reaction (PCR) testing are also performed. For the 2016-2017 season, hospital and public health laboratories submitting RSV data are located throughout the state.
DSHS Laboratory – Microbiological Sciences Branch Notice: Request for Submission of Norovirus Specimens to the Texas DSHS Austin Laboratory.
Testing case patients for unknown gastrointestinal illnesses is helpful for clinical decision making and for community outbreak detection and investigation purposes. Therefore, the Department of State Health Services (DSHS) Laboratory is available to test and sequence gastrointestinal pathogens, including norovirus (NoV). So far in 2022, 12 suspected and confirmed NoV outbreaks have been reported to the DSHS Central Office. Since NoV outbreaks tend to be underreported to public health officials, the true incidence of cases is likely higher.
During outbreak investigations, local or regional health departments may request that you forward clinical specimens to the DSHS Laboratory for additional NoV testing. In such situations, it is essential you submit the requested specimens to the Laboratory to assist epidemiology teams across the state in linking individual cases during outbreak investigations. The DSHS Laboratory does not charge for the testing of clinical specimens that are requested for NoV outbreak investigation purposes.
Appropriate Specimen Type, Packaging, and Shipping
- Required specimens for NoV testing are fresh stool specimens in sterile containers.
- A minimum sample of 200 µl is required for RT-PCR testing.
- Raw stool specimens should be stored cold at 4 °C and shipped overnight in insulated containers with cold packs or wet ice. Do not freeze the specimens.
- Please ensure at least two unique patient identifiers are on the specimen container and its accompanying submission form. Both sets of unique identifiers must be identical.
Norovirus Sample Submission Process
- Norovirus specimens must be accompanied by a G-2B Specimen Submission Form. A sample G-2B form is attached to this notice in which the fields required for norovirus stool specimens are highlighted green. Optional fields are highlighted orange.
- All required fields in the G-2B form must be completed. Do not submit old forms with your specimens. If your facility needs an updated G-2B submission form, please email LabInfo@dshs.texas.gov.
- All submitters must have a submitter ID number with DSHS. If you do not already have an account or submitter number with the DSHS Laboratory, please contact the laboratory at LabInfo@dshs.gov before submitting specimens.
Shipping Information & Address:
https://www.dshs.texas.gov/lab/mrs_shipping.shtm#Shipping
Shipping Addresses:
For Regular Mail Services
Specimen Receiving: Walter Douglass
Laboratory Services Section, MC 1947
Department of State Health Services
PO Box 149347 1100 W. 49th Street
Austin, TX 78714-9347
For Overnight/Priority Courier Service
Specimen Receiving: Walter Douglass
Laboratory Services Section, MC 1947
Department of State Health Services
1100 W. 49th Street
Austin, TX 78756-3199
Phone: 512-776-7569
For laboratory questions about NoV testing or specimen suitability, please contact the Molecular Biology Team Lead at (512) 776-6510. For other questions related to NoV submissions and DSHS’ NoV surveillance project, please contact Kenneth Davis at (512) 921-5368 or Email FoodborneTexas@dshs.texas.gov.
Antimicrobial Stewardship Regional Advisory Committee Membership Update
The Department of State Health Services’ Healthcare Safety Unit is seeking members for Antimicrobial Stewardship Regional Advisory Committees to address antimicrobial stewardship in long-term care facilities and to improve antimicrobial stewardship through collaborative action. DSHS has extended the application deadline for Public Health Region 4/5N to April 15, 2022 at 5:00 p.m.
Each committee will include:
- Physicians
- Directors of Nursing or equivalent consultants with long-term care facilities
- Public health officials knowledgeable about antibiotic stewardship
- Other interested parties, including pharmacists and microbiologists
The Antimicrobial Stewardship Regional Advisory Committees are governed by House Bill 1848 (86th Legislature, Regular Session, 2019).
If you would like to be a founding member of the Antimicrobial Stewardship Regional Advisory Committee in Public Health Region 4/5N, please fill out this application. DSHS will not consider an application, received or postmarked, after April 15, 2022 at 5:00 p.m. Individuals who have already submitted an application do not need to resend.
Meetings will be set during the 2022 calendar year.
For more information, check out our webpage: https://www.dshs.state.tx.us/IDCU/Antimicrobial/Antimicrobial-Stewardship.aspx or contact us at AntibioticStewardship@dshs.texas.gov.
Health and Human Services Commission
GL 22-3002 Chemical Dependency Treatment Facility Requirements During the COVID-19 Pandemic
GL 22-3002 related to the adoption of emergency CDTF requirements due to the COVID-19 pandemic has been posted.
Under the emergency rules, a licensed CDTF may temporarily adjust their usual operation requirements related to telehealth and telemedicine, maximum counselor caseloads, service delivery through two-way, real-time internet or telephone communications, documentation deadlines, and treatment planning and service provision.
These temporary adjustments are designed to assist with limiting the spread of COVID-19, reduce barriers to treatment, and address staffing shortages in response to the COVID-19 pandemic.
Please read the letter at the link below and if you have questions, please contact HCR_PRU@hhs.texas.gov.
HHSC Launches Individualized Skills and Socialization Portal Information Page
HHSC launched a new Individualized Skills and Socialization Services Provider Portal. This new portal allows providers access to information on Individualized Skills Socialization services. Including:
- Complete and review trainings.
- Find and review provider letters and other information and releases.
- Link to rules and other services.
COVID-19 ALF Provider Mitigation and Response Emergency Rule Webinar with LTCR Assisted Living Facility providers should attend this and all Long-term Care Regulation ALF webinars. LTCR provides the latest information and answers questions about the revised COVID-19 Mitigation and Response Emergency rule.
Provider attendance is critical to staying current with requirements and guidance.
ALF Provider Webinar
April 14, 2022
10 – 11 a.m.
Register for the LTCR webinar.
April 18 Deadline for Informal Comments on PPECC Draft Rules
HHSC Long-term Care Regulation is accepting comments from stakeholders on draft rules for prescribed pediatric extended care centers. The comment period ends on April 18, 2022.
Email comments to Long-term Care Regulation Policy and Rules with “22R042” in the subject line.
Deadline for Informal Comments on HHS Draft Rules April 18
Texas Health and Human Services Commission (HHSC) is accepting comments from stakeholders on the following draft rules. The comment period ends to April 18, 2022.
- Texas Administrative Code, Title 26, Part 1, Chapter 550, Sections 550.5, 550.309, 550.1102, and 550.1208, concerning Prescribed Pediatric Extended Care Center Nursing Requirements. Comments can be emailed to HHSC Long-Term Care Regulation.
Questions can be emailed to HHS Rules Coordination Office.
Members Sought for Texas Brain Injury Advisory Council
If you’re interested in issues surrounding brain injury, you may want to apply to be a member of the Texas Brain Injury Advisory Council. The Texas Health and Human Services Commission executive commissioner will appoint members to the council to serve a term expiring Dec. 31, 2024 or Dec. 31, 2025.
Applications are due by 11:59 p.m., April 30.
HHSC will consider the applicants’ qualifications, background and interest in serving on the council and will try to choose council members who represent the diversity of all Texans. For this reason, HHSC considers applicants’ ethnicity, gender and geographic location.
- Review the Texas Brain Injury Advisory Council Application for Membership application letter to find out who the council is seeking as representatives and if you qualify.
- After reviewing the application letter and verifying you meet the qualifications for at least one category, click on the application to apply to be on the council.
A council member must regularly take part in council meetings. They may also have to take part in subcommittee meetings or other related activities. Council meetings are held about once every three months in Austin or at the call of the presiding officer. To the extent permitted by the current state budget, a council member who is serving in the role of a brain injury survivor or a family member actively involved in the care of a loved one who has sustained a brain injury may be repaid for their travel expenses to and from meetings if money is available and in accordance with the HHSC Travel Policy. Council members serving in a professional role aren’t paid to attend or travel to and from council meetings.
HHSC prefers that you submit your application electronically, but you may submit the application by email, mail or fax to the following addresses:
Email: HHS_Appointments@hhs.texas.gov Subject: TBIAC
Mail: Texas Health and Human Services Commission 701 W 51st Street Mail Code 0223 Austin, TX 78751 Attn: Susanna Sparkman
Fax: 512-206-3984 Attn: TBIAC
COVID-19 Mitigation, Response Rule Revised Effective April 3 for ALFs
HHSC Long-term Care Regulation has published a revised assisted living facility COVID-19 Mitigation and Response Emergency Rule (PDF). It is effective April 3, 2022.
The revised rule:
- Defines the term “up-to-date”.
- Removes the definition of personal protective equipment and specific references to the CDC.
- Removes the requirements to have plans to get and maintain a certain amount of PPE.
- Removes the requirement to document staff and visitor screenings.
- Removes the requirement to screen residents at least once a day.
- Uses the term “notify” instead of “report” for contacting HHSC regarding a confirmed case of COVID-19.
Office of Inspector General
Quarterly Report released
The OIG released its Quarterly Report for the second quarter of fiscal year 2022. The agency recovered $86 million, identified another $222 million for potential future recovery, and achieved $41 million in cost avoidance. Click here for the report.
OIG audits residential provider to determine compliance with health and safety rules
The OIG audit team made unannounced visits to the Medicaid provider to learn whether their three- to four-person residences provided clients with a safe and healthy living environment. Read the full audit online.
OIG educates home health care providers
The OIG released information for personal care attendants, describing the common errors that occur when using the Electronic Visit Verification system. Find more information on the OIG website.
Texas Department of Insurance
No Report This week
State Auditor’s Office
No Report this week
Legislative Budget Board
On Friday, March 11, 2022, President Biden signed a $1.5 trillion Omnibus Bill (H.R. 2471), which consists of all 12 appropriations bills for federal fiscal year 2022 (FFY 2022), including $13.6 billion in supplemental funding to provide emergency aid to Ukraine. Prior to the passage of the bill, federal agencies had been operating with appropriations funded through multiple continuing resolutions (CR). This funding is inclusive of any funding federal agencies had already received in the CRs. The bill funds mandatory and discretionary programs across all federal agencies for FFY 2022, excluding amounts already appropriated in COVID-19-related relief legislation and the Infrastructure Investment and Jobs Act. Texas is expected to receive an estimated $14.5 billion (8.1 percent of the national total), distributed among the largest entitlement and discretionary federally funded programs. This amount is an 8.3 percent increase from FFY 2021 funding. Figure 1 shows the estimated fiscal impact to Texas for select programs. Based on information provided by FFIS, the following sections provide a high-level summary for select programs and funding.
EDUCATION K–12 Education: Funding for public education programs in Texas increased by $181.6 million for FFY 2022. The bill funds the Student Support and Academic Enrichment program at $1.3 billion nationally, of which Texas is estimated to receive $125.0 million (an increase of 5.0 percent). Funding for Title I Grants to Local Education Agencies increased by $1.0 billion to $17.5 billion nationally. Of this amount, Texas is estimated to receive $1.7 billion, or approximately 9.6 percent of the total appropriated funding. Special Education-Basic State Grants increased to $13.3 billion nationally for FFY 2022, including $9.0 million for accessible education materials for students with visual and print disabilities. Texas is estimated to receive $1.2 billion (an increase of 3.0 percent). Higher Education: Funding for Career and Technical Education – State Grants increased by 3.3 percent nationally, of which Texas is expected to receive $122.7 million for FFY 2022.
HEALTH AND HUMAN SERVICES State funding for the health and human services functions increased by $114.9 million in FFY 2022. Children’s Health Insurance Program (CHIP) funding increased 5.0 percent to $19.0 billion nationally. CHIP funding for Texas is expected to increase to $1.4 billion. The bill maintains funding from FFY 2021 for both the Social Services Block Grant (SSBG) and Temporary Assistance for Needy Families (TANF) programs. Texas is estimated to receive $142.0 million in SSBG funds and $484.7 million in TANF funds for FFY 2022. The bill extends funding for TANF through September 30, 2022. It also amended the Social Security Act to direct states to provide certain information to victims of sexual harassment and domestic violence through the TANF program. Funding for the Child Care and Development Fund increased by 4.3 percent nationally from FFY 2021. Texas is estimated to receive $673.0 million for FFY 2022, an increase of 5.4 percent. Mental Health and Substance Abuse: Funding for Mental Health Block Grants increased by $100.0 million nationally. Texas is estimated to receive $73.5 million for FFY 2022, an increase of 13.2 percent. The bill maintains Texas funding for State Opioid Response Grants at $52.0 million for FFY 2022. Funding for Substance Abuse and Prevention Block Grant is increased to $1.9 billion nationally, of which Texas is estimated to receive $148.8 million.
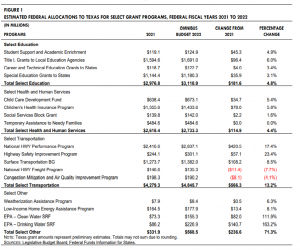
HOUSING Housing. National funding for the Weatherization Assistance Program increased $19.0 million for FFY 2022, of which Texas is estimated to receive $8.4 million, an increase of 6.4 percent. Funding for the Low-Income Home Energy Assistance Program increased by $150.0 million nationally for FFY 2022, of which Texas is estimated to receive $177.9 million (an increase of 8.0 percent), excluding emergency contingency funds. 7485_Final_Summary_Enacted_FFY22_Budget.pdf (texas.gov)
Health Resources and Services Administration
Health Resources and Services Administration Announces Availability of New Funding to Support Community-Based Doulas. Funding will Expand the Community-based Doula Workforce to Help Address Maternal Mortality
The U.S. Department of Health and Human Services (HHS), through the Health Resources and Services Administration (HRSA), announced the availability of $4.5 million for hiring, training, certifying, and compensating community-based doulas in areas with high rates of adverse maternal and infant health outcomes. This announcement builds on the Biden-Harris Administration’s commitment to reduce maternal mortality and morbidity and address the nation’s Black maternal health crisis.
This funding, provided through HRSA’s Healthy Start Initiative, will increase the total number of Healthy Start doula programs from 25 to approximately 50 nationwide. HRSA’s Healthy Start Initiative works to improve health before, during, and after pregnancy and reduce racial and ethnic disparities in rates of infant deaths and adverse maternal health outcomes.
Read the press release.
Provider Relief Fund – Request to Report Late Due to Extenuating Circumstances
| Provider Relief Fund recipients will be able to submit a Request to Report Late Due to Extenuating Circumstances for Reporting Period 1 from Monday, April 11 to Friday, April 22 at 11:59 p.m. ET.
The Request Form will be available starting on Monday, April 11. |
HRSA Seeks Candidates for Advisory Committee on Heritable Disorders in Newborns and Children HRSA is seeking nominations of qualified candidates to serve as voting members of the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC), as well as representatives from relevant organizations.
ACHDNC provides advice, recommendations, and technical information about heritable disorders and newborn and childhood screening to the Secretary of HHS. Members review and report on newborn and childhood screening practices for heritable disorders and recommend program improvements and conditions to include in the Recommended Uniform Screening Panel (RUSP). Representatives are non-voting liaisons from organizations such as national public health constituencies, medical professional societies, and have broad constituencies and interest in newborn screening.
Nominees for members can include medical or scientific professionals with expertise in heritable disorders, screening, counseling, testing, or special services for newborns with heritable disorders, as well as in the ethics of newborn screening. The Committee will appoint two members to serve a term of up to 4 years beginning in 2023. Members receive a stipend and reimbursement for ACHDNC meeting expenses. Nominations for members are due by Monday, April 11 and by Monday, May 2 for organizational liaisons. Federal Register Notices provide more information about nominating a member or a representative.
National Telehealth Conference May 16-17 Join us for the inaugural National Telehealth Conference on May 16-17. Experts and leaders in the field will examine the evolution of telehealth, discuss its place in an integrated health care delivery model, and review the lessons learned during the COVID-19 pandemic to inform the future of telehealth.
Telebehavioral Health Policy Update The January 2020 Federal Public Health Emergency (PHE) declaration for the COVID-19 pandemic led to the temporary expansion of behavioral health service delivery models offered through telehealth, also known as telebehavioral health.
Telebehavioral health has the potential to improve continuity of care for communities by increasing access to a wider network of behavioral health professionals, reducing stigma, and creating spaces for increased engagement and retention in substance abuse treatments. This Association of State and Territorial Health Officials (ASTHO) blog summarizes how Federal and state policy changes are increasing virtual access to behavioral health care.
HRSA’s National Organizations of State and Local Officials Cooperative Agreement provided support for this ASTHO blog.
SUD Value-Based Payment Toolkit As overdose fatalities increase, reaching a historic peak of over 100,000 deaths in 2020, states are continuing to invest in strategies to improve access to treatment for individuals with substance use disorder (SUD). This rise highlights the issues posed by the COVID-19 pandemic to SUD providers. Disruptions and changes in service delivery, and resulting changes in payment, have posed challenges to efforts to increase access to treatment. Policymakers in some states are starting to use the payments they make to providers and managed care plans as effective levers to increase both treatment access and service quality in their Medicaid programs. This National Academy for State Health Policy (NASHP) toolkit examines Medicaid payment strategies that four states use to improve SUD treatment for Medicaid beneficiaries.
HRSA’s National Organizations of State and Local Officials Cooperative Agreement provided support for this NASHP toolkit.
Centers for Medicare and Medicaid Services
FAQs for providers about the No Surprises rules. CMS posted updated Frequently asked Questions for providers about the No Surprises rules. Information for providers and facilities regarding No Surprises rules, independent dispute resolution, and exceptions to the new rules and requirements can be found at: Frequently asked questions for providers about the No Surprises rules. (PDF)
Improve the Health of Minority Populations with Covered Preventive Services and Other Updates
- Fiscal Year 2021 Program for Evaluating Payment Patterns Electronic Reports
- Preventive Services & Health Equity: Improve the Health of Minority Populations
- April 2022 Integrated Outpatient Code Editor (I/OCE) Specifications Version 23.1
- Claim Status Category and Claim Status Codes Update
- Mental Health Visits via Telecommunications for Rural Health Clinics & Federally Qualified Health Centers
- Update to Chapter 7, “Home Health Services,” of the Medicare Benefit Policy Manual (Pub 100-02)
- April 2022 Update of the Hospital Outpatient Prospective Payment System (OPPS)
- Remittance Advice Remark Code (RARC), Claims Adjustment Reason Code (CARC), Medicare Remit Easy Print (MREP) and PC Print Update
- Claims Processing Instructions for the New Pneumococcal 15-valent Conjugate Vaccine Code 90671 and Pneumococcal 20-valent Conjugate Vaccine Code 90677 — Revised
Eligible Individuals Can Receive Second COVID-19 Booster Shot at No Cost
On April 6, CMS announced it will pay for a second COVID-19 booster shot of either the Pfizer-BioNTech or Moderna COVID-19 vaccines without cost sharing, as it continues to provide coverage for this critical protection from the virus. People with Medicare pay nothing to receive a COVID-19 vaccine, and there is no applicable copayment, coinsurance, or deductible. People with Medicaid coverage can also get COVID-19 vaccines, including boosters, at no cost.
The CDC recently updated its recommendations regarding COVID-19 vaccinations. Certain immunocompromised individuals and people ages 50 years and older who received an initial booster dose at least 4 months ago are eligible for another booster to increase their protection against severe disease from COVID-19. Additionally, the CDC recommends that adults who received a primary vaccine and booster dose of Johnson & Johnson’s Janssen COVID-19 vaccine at least 4 months ago can receive a second booster dose of a Pfizer-BioNTech or Moderna COVID-19 vaccine.
The COVID-19 vaccine, including the booster doses, is the best defense against severe illness, hospitalization, and death from the virus. CMS continues to explore ways to ensure maximum access to COVID-19 vaccinations. More information regarding the CDC COVID-19 Vaccination Program Provider Requirements and how the COVID-19 vaccine is provided through that program at no cost to recipients is available at https://www.cdc.gov/vaccines/covid-19/vaccination-provider-support.html and through the CMS COVID-19 Provider Toolkit.
People can visit vaccines.gov (English) or vacunas.gov (Spanish) to search for vaccines nearby.
Centers for Medicare & Medicaid Services Inpatient Rehabilitation Hospital Patient Assessment Instrument (IRF-PAI) Manual, Version 4.0
The Centers for Medicare & Medicaid Services (CMS) is publishing the Inpatient Rehabilitation Facility Patient Assessment Instrument (IRF-PAI) Manual, Version 4.0, effective October 1, 2022. A Change Table outlining the revisions to the CMS IRF-PAI Manual Version 4.0 is also published. The Manual and Change Table can be accessed via the Downloads section IRF-PAI and IRF QRP Manual webpage.
Now Available on eCQI Resource Center: NEW Digital Quality Measurement Page The Electronic Clinical Quality Improvement (eCQI) Resource Center now includes a Digital Quality Measurement page. The Digital Quality Measurement page serves as a centralized location for information on the Centers for Medicare & Medicaid Services’s (CMS) Digital Quality Measurement Strategic Roadmap, and related activities to advancing digital quality measurement. This page aims to keep stakeholders updated on CMS’s goal of transitioning all quality measures used in its reporting programs to digital quality measures (dQMs).
On the webpage you will find:
- A brief overview of CMS’s Digital Quality Measurement Strategic Roadmap,
- Information on dQMs,
- Associated documentation, and
- Tools and resources (including related initiatives and projects)
CMS encourages users to explore the new Digital Quality Measurement page.
Attention Home Health Providers We had previously identified an issue in the iQIES system that may have affected your HHA’s Services Provided values that are displayed on the HHA Provider Preview reports that were distributed in iQIES on 02/23/2022. Moreover, we had previously asked HHAs to review the Services Provided Information on the HHA Provider Preview Reports for the April 2022 refresh and contact their OASIS Education Coordinator (OEC) or OASIS Automation Coordinator, should inaccuracies be identified. The process to collect and maintain the Services Provided information recently migrated into iQIES and we are addressing the issues identified.
To allow time to complete the identified changes, we have decided for the April 2022 refresh, to publish the same Services Provided data for each Home Health Agency that was posted on Care Compare for the January 2022 refresh. We believe that this will allow us to publish the most accurate Services Provided data at this time.
We are still urging HHA providers to carefully review the Services Provided data within the recently issued Preview Reports, however, we would like to note that we are not attaching a deadline to this review. These reports were distributed in iQIES on 02/23/2022. If the values are incorrect for your agency, please contact your State Automation or State OEC and request an update of your Services Provided data in iQIES. Should you have questions, please contact the iQIES Help Desk by phone at (800) 339-9313 or by email at iQIES@cms.hhs.gov. CMS continues to work to refine and rectify the update process moving forward.
For those experiencing issues locating your agency’s HHA Provider Preview Report, follow the steps outlined below:
New Users
- Only active users with login credentials to iQIES as of the distribution date of the HHA Provider Preview reports for the April 2022 refresh will have the report in their HHA Provider Preview Report folder.a. If you are a new user of iQIES and received your iQIES login credentials after the February 23, 2022 distribution of the HHA Provider Preview reports, please contact the iQIES Help Desk for assistance (see iQIES Help Desk contact information below).
b. Inform the help desk staff that you are a new user of iQIES since the HHA Provider Preview reports for the April 2022 refresh were made available. The iQIES help desk team will gather the necessary information from you including such things as your agency’s CMS Certification Number (CCN) and will then make the report for your agency available to you in the HHA Provider Preview Reports folder in iQIES.
c. Follow the steps below to locate your agency’s report in the HHA Provider Preview Report folder.
Existing Users
- If you were an active user prior to the February 23, 2022 distribution of the HHA Provider Preview Reports but you cannot locate your report, please follow the steps below:a. Log into iQIES at https://iqies.cms.gov/.
b. Select the My Reports option from the Reports menu
c. From the My Reports page, locate the HHA Provider Preview Reports folder.
i. NOTE: The folders and reports on the My Reports page are listed in alphabetic order so users may need to utilize the page forward functionality at the bottom of the webpage to advance to the page where the folder is located. Alternatively, users may change the default number of rows that display on the webpage from 10 to a larger number to view the larger list of items.
d. Select the HHA Provider Preview Reports link to open the folder.
e. To locate the latest HHA Provider Preview report, select the down arrow adjacent to the Created Date label at the top of the table. This will order the reports in the folder from newest to oldest.
f. Select the report file with the following label: Preview of Home Health Agency Quality Measure Scores To Be Posted on Care Compare (April 2022)_Updated [provider’s CCN will display after the word Updated].
g. Once the report is open, users will notice the Services Provided information displayed beneath their agency’s demographic information at the top of the report.
Should you have questions, please contact the iQIES Help Desk by phone at (800) 339-9313 or by email at iQIES@cms.hhs.gov.
2023 Medicare Advantage and Part D Rate Announcement
The Centers for Medicare & Medicaid Services (CMS) released the Announcement of Calendar Year (CY) 2023 Medicare Advantage (MA) Capitation Rates and Part C and Part D Payment Policies (the Rate Announcement). CMS’s goals for Medicare Advantage and Part D mirror our vision for the agency’s programs as a whole, which is to advance health equity; drive comprehensive, person-centered care; and promote affordability and the sustainability of the Medicare program.
In the CY 2023 MA and Part D Advance Notice, CMS solicited comments on a variety of topics, including seeking input on promoting health equity in Medicare Advantage and Part D plans. CMS appreciates the submitted comments and will consider them in future policymaking.
This fact sheet discusses the provisions of the Rate Announcement, which can be viewed by going to: https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Announcements-and-Documents.html and selecting “2023 Announcement.”
Net Payment Impact
The chart below indicates the expected impact of the policy changes and updates on MA plan payments relative to 2022.
Year-to-Year Percentage Change in Payment
| Impact | 2023 Advance
Notice |
2023 Rate
Announcement |
| Effective Growth Rate | 4.75% | 4.88% |
| Rebasing/Re-pricing | N/A[1] | 0.39% |
| Change in Star Ratings | 0.54% | 0.54% |
| Medicare Advantage Coding Pattern Adjustment | 0% | 0% |
| Risk Model Revision | 0% | 0% |
| Normalization | -0.81% | -0.81% |
| MA risk score trend[2] | 3.50% | 3.50% |
| Expected Average Change in Revenue | 7.98 % | 8.50% |
Part C Risk Adjustment
CMS will continue the CY 2022 policy to calculate 100% of the risk score using the 2020 CMS-HCC model, which was phased in from CY 2020 to CY 2022, as required by section 1853(a)(1)(I) of the Social Security Act, as amended by the 21st Century Cures Act. We are also continuing our policy of calculating risk scores for MA enrollees using diagnoses exclusively from MA encounter data submissions and fee-for-service (FFS) claims. CMS solicited and received comments on whether enhancements can be made to the CMS-HCC risk adjustment model to address the impacts of social determinants of health on beneficiary health status by incorporating additional factors that predict the relative costs of MA enrollees and will consider all comments received on this topic for future policymaking.
Part C End Stage Renal Disease (ESRD) Risk Adjustment
For CY 2023, we are finalizing the revised risk adjustment model for payment to MA organizations and additional demonstrations and programs (such as Medicare-Medicaid Plans (MMPs)) where the demonstration also uses the MA risk adjustment models) for enrollees with ESRD in order to improve the prediction of costs for these enrollees. The revised model is calibrated on more recent data, using CMS’s current approach to identify risk adjustment eligible diagnoses from encounter data records. It also incorporates improvements previously made to the Part C CMS-HCC model, specifically the clinical updates and revised segmentation, which accounts for the differential cost patterns of dually eligible beneficiaries.
Program of All-Inclusive Care for the Elderly (PACE) Risk Adjustment
For CY 2023 payment to PACE organizations, we will continue to use the 2017 CMS-HCC model to calculate non-ESRD risk scores as we have done since CY 2020, the 2019 CMS-HCC ESRD models to calculate ESRD risk scores as we have done since CY 2019, and the 2020 RxHCC model to calculate Part D risk scores as we have done since CY 2020.
Medicare Advantage Coding Pattern Adjustment
Each year, as required by law, CMS makes an adjustment to plan payments to reflect differences in diagnosis coding between MA organizations and FFS providers. For CY 2023, CMS is finalizing a coding pattern adjustment of 5.9%, which is the minimum adjustment for coding pattern differences required by statute. CMS received a number of recommendations from stakeholders regarding approaches to estimate the MA coding pattern adjustment. These included recommendations that CMS apply a higher coding pattern adjustment than the statutory minimum and that CMS consider approaches that take into account differences in coding patterns across MA plans. CMS continually reviews MA coding patterns and continues to assess how we calculate the MA coding pattern adjustment, how best to apply it, and what the appropriate level of the adjustment should be. Ensuring that the coding pattern adjustment policy appropriately addresses differential coding in MA is essential and we will consider these recommendations in the development of future coding pattern adjustment proposals.
Medicare Advantage Normalization Factor
CMS calculates normalization factors annually to keep the FFS risk score at the same average level over time. For CY 2023, CMS will use the methodology typically used for calculating the normalization factor, which is to project the payment year risk score using a trend that is based on five historical years of FFS risk scores under the payment year model. However, for CY 2023, we proposed not to update the years of FFS risk scores used in the trend as we typically do – that is to remove the earliest year’s FFS risk score and add the most recent year’s FFS risk score that is available – because of concerns that the changing use of services in 2020 due to the COVID-19 pandemic resulted in an anomalous 2021 risk score, which is based on diagnoses from 2020 dates of service. Including the anomalous 2021 risk score would result in a projection that significantly underestimates what the FFS 2023 risk score is likely to be. CMS is finalizing the proposal to not update the years in the trend and instead use the same years of FFS risk scores that were used to calculate the 2022 normalization factors, 2016 through 2020.
Part D Risk Adjustment
For CY 2023, we are finalizing our proposal to implement an updated version of the RxHCC risk adjustment model for Part D sponsors other than PACE. The RxHCC model is used to adjust direct subsidy payments for Part D benefits offered by stand-alone prescription drug plans (PDPs) and Medicare Advantage prescription drug plans (MA-PDs). The recalibrated RxHCC model includes a clinical update to the RxHCCs based on ICD-10-CM diagnosis codes rather than ICD-9-CM codes used in the prior models. The recalibrated model also includes an update to the data years (2018 diagnoses to predict 2019 costs) using the same approach we use to filter diagnoses from encounter data records for risk score calculation, including the risk adjustment allowable CPT/HCPCS codes.
Puerto Rico
The proportion of Medicare beneficiaries who receive benefits through the MA program (as opposed to FFS Medicare) is far greater in Puerto Rico than in any other state or territory. The policies proposed and finalized for 2023 will continue to provide stability for the MA program in the Commonwealth and to Puerto Ricans enrolled in MA plans. These policies include basing the MA county rates in Puerto Rico on the relatively higher costs of beneficiaries in FFS who have both Medicare Parts A and B, continuing the statutory interpretation that permits certain counties in Puerto Rico to qualify for an increased quality bonus adjusted benchmark, and applying an adjustment to reflect the nationwide propensity of beneficiaries with zero claims.
Part C and D Star Ratings
The Rate Announcement includes information and announces updates in accordance with the Star Ratings regulations at §§ 422.164, 422.166, 423.184, and 423.186.
The Rate Announcement includes information about the date by which plans must submit their requests for review of the appeals and complaints measures data, lists the measures included in the Part C and D Improvement measures and the Categorical Adjustment Index for the 2023 Star Ratings, and lists the states and territories with Individual Assistance designations that began in 2021 from the nationwide FEMA major disaster declarations used in the definition of an affected contract for the extreme and uncontrollable circumstances adjustment for the 2023 Star Ratings.
Additionally, CMS solicited feedback in the CY 2023 Advance Notice on a number of different potential measurement concepts and methodological enhancements, including the following:
- Plans to enhance current CMS efforts to report stratified Part C and D Star Ratings measures by social risk factors to help MA and Part D sponsors identify opportunities for improvement. Nearly all stakeholders supported stratified reporting, and we will begin sharing confidential stratified reports with contracts this spring.
- The development of a Health Equity Index as an enhancement to the Part C and D Star Ratings program to summarize measure-level performance by social risk factors into a single score used in developing the overall or summary Star Rating for a contract.
- The development of a measure to assess whether plans are screening their enrollees for health-related social needs such as food, housing, and transportation.
- How MA organizations are transforming care and driving quality through value-based contracts with providers to use in the potential development of a Part C Star Ratings measure.
CMS will take the feedback received into consideration as we continue to explore ways to further drive health equity and high quality care.
Modified Sections of the Inpatient Rehabilitation Facility Patient Assessment Instrument (IRF-PAI) The new and modified sections of the IRF-PAI, effective October 1, 2023 proposed for the IRF QRP in the FY 2023 IRF PPS Proposed Rule (CMS-1767-P), can be found in the Downloads section on the IRF-PAI and IRF-PAI Manual webpage.
Updated Group Health Plan (GHP) Correspondence Cover Sheet Now Available
An updated GHP Correspondence Cover Sheet is now available in the Download section of the Group Health Plan Recovery page on CMS.gov. Please remember to use this cover sheet when sending GHP recovery related correspondence to the Commercial Repayment Center (CRC).
Congressional Budget Office
Estimated Budgetary Effects of the Bipartisan COVID Supplemental Appropriations Act, 2022 Title I of the legislation would provide funding for federal agencies to prevent, prepare for, and respond to the coronavirus pandemic. Title II would rescind or repurpose about $10,465 million in unobligated balances from a variety of accounts and specify requirements for the budgetary treatment of section 1206 and sections 1209 through 1211. In keeping with those requirements, and at the direction of the Senate Committee on the Budget, those four sections are considered authorizing legislation rather than appropriation legislation. As a result, the estimated budgetary effects of section 1206 and sections 1209 through 1211 are subject to pay-as-you-go procedures. However, section 1206 also requires the estimated budgetary effects stemming from that section to be excluded from the pay-as-you-go scorecards maintained by the Senate and the Office of Management and Budget.
Section 1204 would designate the budgetary effects of the legislation as emergency requirements, in keeping with section 4001(a)(1) and section 4001(b) of S. Con. Res. 14 (117th Congress), the concurrent resolution on the budget for fiscal year 2022. In consultation with the Senate Budget Committee, that designation would not apply to section 1206 or sections 1209 through 1211. CBO Estimate for the Bipartisan COVID Supplemental Appropriations Act, 2022
US Food and Drug Administration
No Report This week
National Institutes of Health
COSWD Strategic Plan for Fiscal Years 2022–2026 Now Available
I am pleased to present the Chief Officer for Scientific Workforce Diversity (COSWD) Strategic Plan for Fiscal Years 2022–2026. It outlines a bold vision for our future efforts and renews our charge to lead the science of scientific workforce diversity, using evidence-based approaches to catalyze cultures of inclusive excellence in support of the NIH mission.
As the United States confronts challenges and threats to equity, my office is advancing its mission to address these issues in science. Our new strategic plan is grounded in our continued commitment to enable the NIH and NIH-funded institutions to benefit from the full range of talent in the nation, fostering creativity and innovation in science.
Guided by the plan, our activities over the next five years will promote diversity, equity, inclusion, and accessibility (DEIA) in the biomedical research enterprise by focusing on three related goals:
- Goal 1: Build the evidence using research insights and the NIH as a testbed for innovative scientific programs to enhance diversity in the workforce.
- Goal 2: Disseminate the evidence through work with the biomedical scientific community, from trainees to established tenured scientists.
- Goal 3: Act on the evidence by advancing integrated, institution-wide systems to address bias, equity, mentoring, and work-life issues.
The plan describes the objectives and tactics we will employ to achieve these goals and the three cross-cutting strategies—collaborations, accountability, and evaluation—that will guide our pursuit of them.
Our strategic priorities continue the momentum established by the first COSWD strategic plan released in 2016. We intend to scale and accelerate our progress toward a more equitable scientific endeavor by building on prior COSWD accomplishments, including those summarized in our 2021 Year in Review.
Our new plan aligns with the NIH-Wide Strategic Plan for Fiscal Years 2021–2025 and the forthcoming NIH-Wide Diversity, Equity, Inclusion, and Accessibility (DEIA) Strategic Plan. Together, the three efforts present a cohesive vision for fostering cultures of inclusive excellence throughout the biomedical science workforce ecosystem.
I am deeply grateful to the COSWD team for their vital contributions to our strategic plan. In addition, I thank the NIH community and our external stakeholders for sharing their critical insights and feedback throughout the planning process. This input helped us produce a plan that will enhance scientific workforce diversity across the talent lifecycle.
Please explore the strategic plan in full to learn how we will pursue the goal of promoting transformative change. We look forward to partnering across NIH and beyond to foster this change, and I will share our progress as we bring this new vision to life. You can stay up to date with our activities by subscribing to this blog and following us on TwitterExit Link Disclaimer and LinkedInExit Link Disclaimer
NIH funds new tuberculosis research advancement centers The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, today announced four new grant awards to establish Tuberculosis Research Advancement Centers (TRACs). The centers will support the development of a next generation of tuberculosis (TB) researchers by providing focused mentoring and funding support for new investigators; opportunities for multidisciplinary and collaborative research; and training in laboratory and clinical settings. The total funding in the first year of these five-year grants is approximately $4.3 million.
TB is a bacterial disease that currently is the second leading cause of death, after COVID-19, from a single infectious agent worldwide. In 2020, as many as 10 million new TB cases were diagnosed and approximately 1.5 million lives were lost to the disease. Alleviating the global burden of TB through research to discover or improve diagnostics, therapeutics and vaccines is a top priority for NIAID, as outlined in the 2018 Strategic Plan for Tuberculosis Research.
Awards have been made to the following institutions:
Texas Biomedical Research Institute, San Antonio
Principal Investigator: Larry Schlesinger, M.D.
Grant P30AI168439-01
The Interdisciplinary NexGen TB Research Advancement Center (IN-TRAC) will support career development, and provide experiential training in biosafety and biocontainment training, animal models, and animal imaging modalities. Participants will be trained in TB clinical research and TB patient care at the only free-standing TB hospital in the United States (Texas Center for Infectious Disease, San Antonio) and at clinics and field sites along the Texas-Mexico border.
Johns Hopkins University, Baltimore
Principal Investigators: Petros Karakousis, M.D., and Richard Chaisson, M.D.
Grant P30AI18436-01
Working with partners and collaborators at clinical sites in Baltimore and internationally, the Johns Hopkins University (JHU) TRAC will enhance the integration, productivity and impact of JHU’s existing TB research programs and foster career development. The JHU TRAC will also support clinical, basic and computational TB research through services provided via four research cores: a clinical core; a microbiology, immunology, animal modeling and imaging core; a pharmacology and pharmacometrics core; and a bioinformatics, modeling and biostatistics core.
Emory University, Atlanta
Principal Investigators: Neel Gandhi, M.D., and Jyothi Rengarajan, Ph.D.
Grant P30AI168386-01
The Emory/Georgia TB Research Advancement Center (TRAC) and partner institutions will support career development and provide Center participants with access to study populations in the United States and in countries with a high burden of TB. This TRAC will also provide resources for studies in pathogenesis and host immunity in animal models, including research with non-human primates. It will also offer opportunities to use cutting-edge technologies and integrate systems biology into experimental design.
University of Washington School of Medicine, Seattle
Principal Investigators: Chetan Seshadri, M.D., Rhea Coler, Ph.D., and David Sherman, Ph.D.
Grant P30AI168034-01
The Seattle Tuberculosis Research Advancement Center (SEA-TRAC) will catalyze new avenues of research and train new investigators to make a meaningful impact on the TB epidemic. The Development Core will oversee educational, training and grant programs designed to foster career development of junior or senior investigators who are new to TB research. The Clinical and Translational Science Core will lead training and consulting in clinical research methodology and foster collaborative research with international partners. The Basic Science Core will provide training for scientists new to working in the Biosafety Level-3 (BSL-3) environment and will lead training and consulting in advanced microbiology and immunology methods. The Data Sciences Core will leverage strengths in biostatistics, computational biology and modeling to train scientists who are new to data science and will offer consulting services for advanced research questions.
Saving a Life When No One Is Around. Recognizing the warning signs of an opioid overdose can save a life. A person in this scenario may be unconscious with very small pupils and slow or shallow breathing. They may also be unable to speak and have a faint heartbeat and cold, clammy skin. Their lips and fingernails may look blue or purple.
Overdose is a medical emergency, and fast medical attention can be lifesaving using the highly effective rescue medication naloxone, an opioid blocker. Many states have passed “Good Samaritan” laws that legally protect a person who experiences an overdose, as well as those who call 911 to report the emergency and help the individual.
But because, tragically, most people overdose alone, other solutions are necessary. One currently in development is an implanted device that will automatically detect an overdose and give naloxone. This work is funded by the Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, which is looking for new treatments for opioid use disorder and overdose.
Technology Solutions
The majority of overdoses now involve strong synthetic opioids like fentanyl that work in very small amounts. People who use drugs often do not know exactly what they are taking, and if substances are injected, they take effect very rapidly. Among the most vulnerable to overdose are those who have not used drugs in a while, such as people in recovery or those being released from jails or prisons.
High doses of opioids like heroin or fentanyl can have dangerous side effects because they stick to cells in the part of the brain that controls breathing. Naloxone reverses an opioid overdose by displacing these opioid molecules. Currently, naloxone can be given by injection or through a prefilled, needle-free nasal spray that requires no assembly and is available at pharmacies. Law enforcement and first responders often carry it with them.
A HEAL-funded research team is taking a creative approach that might be able to help individuals experiencing an overdose when no one else is around.
Robert Gereau, Ph.D., of Washington University in St. Louis, Missouri, and John Rogers, Ph.D., of Northwestern University in Evanston, Illinois, are developing a miniaturized overdose-detection device. Implanted under the skin, the device would detect an overdose, automatically administer naloxone, and alert emergency responders and other members of the individual’s support system.
The implant technology works by continuously measuring blood oxygen levels and automatically administering naloxone when oxygen levels get dangerously low, which occurs during an overdose. Although measuring blood oxygen is a common medical procedure – for example, using small devices called oximeters that clip to an individual’s finger or earlobe – these wearable devices are conspicuous and impractical for long-term use in people with opioid use disorder.
If this now-experimental device is approved for use in humans, it will be able to do several things. In addition to measuring blood oxygen levels, the device has a rechargeable battery to power it and a pump with multiple reservoirs of naloxone as a backup in case the first dose isn’t delivered correctly or is not enough.
To be optimally effective, the device will be able to operate independently and communicate if and when a person can’t. The HEAL-funded team is developing a chip connected to a mobile device that can alert emergency responders or others who can help.
So far, this experimental naloxone implant works well in rodents, and researchers will next test it in larger animals, such as pigs, whose body functions more closely resemble those of the human body. Those tests will help determine, for example, the best place in the body to implant such a device and measure oxygen levels.
Other Options
Although technology-based solutions like this naloxone implant will increase the number of tools to keep people healthy and save lives, other options to expand awareness and use of medications like naloxone are being explored.
For example, HEAL-funded research is working on increasing use of naloxone by teaming with communities to reduce stigma about its use. Other strategies include different versions of opioid blockers, novel molecules that work outside the body’s opioid system, and even wearable devices being developed to detect problems with breathing that often precede overdose.
Antibodies recognize new SARS-CoV-2 Omicron variant after booster
At a Glance
- After a booster vaccination, levels of antibodies in the blood that could bind to and neutralize a new Omicron subvariant increased substantially.
- The quick spread of this new subvariant, called BA.2, is likely due to its greater infectiousness rather than its ability to evade the immune system.
Since scientists in South Africa announced its discovery in November 2021, the Omicron variant of SARS-CoV-2 has rapidly become dominant around the world. It currently accounts for almost all COVID-19 cases in the U.S.
Omicron has a large number of mutations in its spike protein. These make it better at latching onto and infecting cells. However, studies have shown that vaccines developed earlier in the pandemic produce an immune response that can recognize and block Omicron after a booster shot. This means that vaccines still help prevent serious illness from Omicron.
But SARS-CoV-2 continues to evolve. Three subvariants of Omicron have been identified to date. The one responsible for the surge in cases that began in late 2021 is called BA.1. Recently, the number of cases driven by a newer subvariant, called BA.2, has increased sharply. It hasn’t been known whether antibodies—produced either by vaccination or previous SARS-CoV-2 infection—can effectively recognize BA.2.
A research team led by Dr. Dan Barouch from Beth Israel Deaconess Medical Center examined neutralizing antibodies produced after vaccination or infection. These are antibodies that can bind to the virus and block its entry into cells.
The team tested antibodies from the blood of 24 people who had been vaccinated and boosted with the Pfizer-BioNTech shot. They also looked at antibodies from 8 people who had recovered from COVID-19, 7 of whom had also been vaccinated.
The study was funded in part by NIH’s National Cancer Institute (NCI). Results were published on March 16, 2022, in the New England Journal of Medicine.
As with the Omicron variant BA.1, the number of neutralizing antibodies in the blood after initial vaccination that could recognize BA.2 was more than 20 times lower than those that could identify the original virus. This number continued to decline six months after vaccination.
However, after the booster dose, levels of antibodies that could neutralize BA.2 jumped substantially. The number that could recognize BA.2—as well as BA.1—after the booster dose was higher than those recognizing the original virus after only the first two shots.
The number of antibodies that could recognize BA.2 were even higher in people who had been vaccinated and infected with the original Omicron variant. The one person who had no detectible neutralizing antibodies in their blood after exposure to SARS-CoV-2 hadn’t been vaccinated.
“These results indicate that the recent increase in COVID-19 cases caused by BA.2 is more likely due to an improved ability of the virus to spread from person to person rather than its ability to evade the immune system,” Barouch notes.
The results, combined with findings from other studies, show that a booster dose prompts the body to makes antibodies capable of neutralizing a range of SARS-CoV-2 variants.
Other Popular Stories:
- SARS-CoV-2 infection of the inner ear
- COVID-19 vaccines linked to small increase in menstrual cycle length
- COVID-19 immune response improves for months after vaccination
- Study suggests Epstein-Barr virus may cause multiple sclerosis
- Blocking hormone improves Alzheimer’s symptoms in mice
A Better Way to Talk About Problems with Alcohol Misuse
Did you know that language commonly used to describe alcohol misuse and alcohol use disorder (AUD) can influence treatment outcomes in people suffering from alcohol problems? Yes, that can often be the case. In fact, the stigma perpetuated by such language can decrease a person’s motivation to seek help for an alcohol problem.
The stigma also can affect one’s self-esteem, as well as how they are perceived by others. And, sadly, AUD is still often viewed as a moral failing or character flaw, rather than a chronic medical disorder from which people can—and do—recover. Less than 10 percent of people with AUD obtain treatment or help for alcohol problems. Reframing the way we talk and think about alcohol problems can encourage people to seek and receive the help they need to recover.
In a recent article published in the journal Neuropsychopharmacology, my NIH colleagues, National Institute on Drug Abuse Director Nora D. Volkow, and National Institute of Mental Health Director Joshua Gordon, and I discuss the impact of stigma on people who have a mental illness or an alcohol or other substance use disorder [1]. We discuss how word choice can perpetuate stigma, leading to lower self-esteem, decreased interest in seeking help, and consequent worsening of symptoms.
We also point to evidence that stigma-related bias among clinicians can contribute to a treatment-averse mindset and to suboptimal clinical care, including failure to implement evidence-based treatment [2]. Studies have shown that the use of clinically accurate language and terms that centralize the experience of patients reduces stigma, resulting in higher quality health care.
Although more evidence-based treatment options for AUD are available today than ever before, stigma is a barrier that prevents some people from accessing treatment. Understanding that AUD is a medical condition and choosing our words carefully when discussing alcohol-related problems are important steps toward changing the conversation. It will empower people to seek treatment for AUD and help clinicians to deliver optimal care.
We can help alleviate the stigma associated with alcohol-related conditions in all settings by consistently using non-pejorative, non-stigmatizing, person-first language to describe such conditions and the people who are affected by them.
Here is some recommended language for reducing alcohol-related stigma that might be helpful:
- Use alcohol use disorder, or AUD, instead of alcohol abuse, alcohol dependence, and alcoholism. In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), AUD replaces the older categories of alcohol abuse and alcohol dependence with the single disorder, AUD, which ranges from mild to severe.
- Use alcohol misuse instead of alcohol abuse when referring broadly to drinking in a manner, situation, amount, or frequency that could cause harm to the person who is engaging in drinking or to those around that person. For some individuals, any alcohol use constitutes alcohol misuse.
- Use person-first language to describe people with alcohol-related problems such as:
- Person with alcohol use disorder instead of alcoholic or addict
- Person in recovery or person in recovery from alcohol use disorder instead of recovering alcoholic
- Person who misuses alcohol or person who engages in alcohol misuse instead of alcohol abuser and drunk
- Use alcohol-associated liver disease instead of alcoholic liver disease. Also use alcohol-associated hepatitis, alcohol-associated cirrhosis, and alcohol-associated pancreatitis instead of alcoholic hepatitis, alcoholic cirrhosis, and alcoholic pancreatitis. The use of “alcoholic” as an adjective may perpetuate stigma for people with alcohol-associated liver disease and other alcohol-related health conditions.
April is Alcohol Awareness Month, which is a good time to think about how alcohol is affecting your life. If you or a loved one need help for alcohol-related problems, the NIAAA Alcohol Treatment Navigator is a one-stop resource for learning about AUD and evidence-based AUD treatment. The Navigator teaches what you need to know and what you need to do to find evidence-based treatment options in your area.

