Upcoming Public Meetings and Events From HHSC: While every effort has been made to offer an accurate and current listing of meeting agendas and events on this calendar, the information has been compiled from a variety of sources and is subject to change without notice to the user.
Please Note: This is a comprehensive list of upcoming meetings provided by HHSC. Not every meeting fits the parameters for coverage by Texas Insight. Refer to Texas Insight’s “Weekly Insight” newsletters for regular updates on planned coverage.
For details on the postings follow this link Communications & Events | Texas Health and Human Services
Designates meetings to be covered by Texas Insight ***
Health and Human Services Commission and Department of State Health Services
April 28, 2022
9:30 am Advisory Committee
April 29, 2022
10:00 am Advisory Committee
12:00 pm Advisory Committee
May 4, 2022
9:00 am Advisory Committee
1:00 pm Advisory Committee
May 5, 2022
9:00 am Advisory Committee
May 6, 2022
9:00 am Advisory Committee
May 13, 2022
9:30 am Advisory Committee
May 16, 2022
9:00 am Public Hearing
9:00 am Public Hearing
9:00 am Public Hearing
Texas House of Representatives
No HHS related public hearings
Texas Senate
No HHS related public hearings
HHSC has the Following Rules Available for Comment. The Administrative Procedure Act (Texas Government Code, Chapter 2001(link is external)) requires the notice published in the Texas Register to include a brief explanation of the proposed rule and a request for comments from any interested person. The notice also includes instructions for submitting comments regarding the rule to the agency, including the date by which comments must be submitted. Agencies must give interested persons “a reasonable opportunity” to submit comments. The public comment period begins on the day after the notice of a proposed rule is published in the Texas Register and lasts for a minimum of 30 calendar days.
The Administrative Procedure Act (Texas Government Code, Chapter 2001(link is external)) requires the notice published in the Texas Register to include a brief explanation of the proposed rule and a request for comments from any interested person. The notice also includes instructions for submitting comments regarding the rule to the agency, including the date by which comments must be submitted. Agencies must give interested persons “a reasonable opportunity” to submit comments. The public comment period begins on the day after the notice of a proposed rule is published in the Texas Register and lasts for a minimum of 30 calendar days. For draft rules detail please see Comment on Proposed & Draft Rules | Texas Health and Human Services or follow the links to each individual rule.
| Title | Project No. / Description | Comment End Date |
| Title 26, Chapter 556, Sections 556.2, 556.3, and 556.5 – 556.13, concerning Nu… | #21R164: NATCEP Clinical Site Expansion | 5/9/22 |
| Title 26, Chapter 745, Sections 745.609, 745.611, 745.613, 745.615, and 745.617… | #21R167: Fingerprint Criminal History Checks | 5/9/22 |
Informal Comments
Informal opportunities to comment occur before a rule is published in the Texas Register. HHS staff may solicit informal public and stakeholder input by:
- inviting stakeholders to submit comments on potential rule changes during rule development.
- sharing a draft rule with stakeholders for review.
- using existing HHS advisory committees to comment on rules.
The following are draft rules on which HHS is accepting informal public or stakeholder input. All rules are posted in MS Word format unless otherwise noted.
No Informal Rule Proposals At This Time
For detail on the rate proposals and older rate proposals please follow the link: Rate Packets | Provider Finance Department (texas.gov) or follow the live link by each proposal.
| Title | Proposed Effective Date | Documents |
| Notice of Proposed Adjustments to Fees, Rates or Charges for Medical Policy Review of Physician Administered Drugs: Vaccines & Toxoids | September 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for Mobility Aids | June 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for Collaborative Care Management Services | June 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates, or Charges for Medicaid Biennial Calendar Fee Review of the following: Long Acting Reversible Contraceptives (LARCs) | April 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates, or Charges for Policy Fee Review of the following: Prosigna | April 01, 2022 |
|
| Notice of Proposed Temporary Rate Actions for American Rescue Plan Act (ARPA) Home and Community-Based Services (HCBS) Provider Retention Payments | March 01, 2022 | · Notice of Proposed Temporary Rate Actions for American Rescue Plan Act (ARPA) Home and Community-Based Services (HCBS) Provider Retention Payments |
| Notice of Proposed Medicaid Payment Rates for the Medical Policy Review of End State Renal Disease Revenue Code 881 | March 01, 2022 |
2022-03-01-pmnt-rates-ersd.pdf
|
| Biennial Calendar Fee Review: Vaccines and Toxoids Q Codes NDCX | March 01, 2022 |
03-01-2022-biennialcalendarfeereview-vaccine-toxoids-qcodes-ndcx.pdf |
| Notice of Proposed Medicaid Payment Rates for the Medical Policy Review of End Stage Renal Dialysis Revenue Codes | March 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for IV Therapy Equipment and Supplies | March 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for Nutritional (Enteral) Products, Supplies, and Equipment – Home Health & CCP: Immobilized Lipase Cartridge | March 01, 2022 |
|
| Proposed Rate Actions for Fiscal Year 2022-23 Quarter 1 Biennial Fee Review | March 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges Healthcare Common Procedure Coding System (HCPCS) | March 01, 2022 |
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for Cardiac Magnetic Resonance Imaging | March 01, 2022 |
|
| Biennial Calendar Fee Review | March 01, 2022 |
03-01-2022-biennialcalendarfeereview.pdf
|
| Notice of Proposed Adjustments to Fees, Rates or Charges for Q Codes | March 01, 2022 |
|
Attorney General
No Report This week
Office of the Governor
Governor Abbott Statement On CMS Decision To Withdraw 1115 Waiver Rescission Governor Greg Abbott today issued a statement after the Centers for Medicare & Medicaid Services (CMS) announced their decision to withdraw their April 16, 2021 letter rescinding the previously approved 1115 Medicaid waiver extension. Last April, the Biden administration rescinded the extension that was granted by the prior administration. This extension, which was scheduled to run through 2030, provides critical healthcare funding, including funds for uncompensated care.
“CMS’ decision to withdraw the rescission of the 1115 Medicaid waiver extension allows Texas’ 1115 Waiver, originally approved in January 2021, to continue for the next 10 years and ensures that Texans will have access to crucial healthcare funding, including funds for uncompensated care. I applaud CMS for their reversal and thank Texas Attorney General Ken Paxton for fighting this decision over the past year in court. I also thank the Texas Health and Human Services Commission for their tireless work to secure this waiver extension. The original rescission by the Biden administration last April obstructed healthcare access for vulnerable Texans and took away crucial resources for rural hospitals in Texas, and I am grateful that Texans will now continue to have access to the health care resources they need.”
The waiver extension allows the state to maintain an efficient and effective Medicaid managed care model while standing up new programs, including those to support the replacement of the Delivery System Reform Incentive Payment (DSRIP) program which ended September 30, 2021. The extension also allows the Texas Health and Human Services Commission to maintain existing directed-payment programs, including the Uniform Hospital Rate Increase Program for hospitals and the Quality Incentive Payment Program for nursing facilities, and to implement new or increased directed-payment programs in managed care contracts as outlined in the DSRIP transition plan and approved by CMS. The current Uncompensated Care program will continue to be authorized at $3.87 billion until resizing for fiscal year 2023. A new Uncompensated Care program launched in October 2021, supports uninsured Texans receiving behavioral health and public health services, providing stability for these services as DSRIP programs phased out.
Department of Family and Protective Services
No Report This week
Department of State Health Services
DSHS COVID Update
Updated Information for COVID-19 Vaccination Providers:
- COVID-19 Vaccine Provider Webinars (last updated 4/19/22)
Main COVID-19 Site Updates:
- COVID-19 Cases and Deaths by Vaccination Status Dashboard (last updated 4/21/22)
- ADDED: Notice of Dashboard Maintenance: The COVID-19 Cases and Deaths by Vaccination Status Dashboard is undergoing scheduled maintenance from Saturday, April 23 at 8:00 am CT through Sunday, April 24 at 5:00 pm. The dashboard will be available again Monday, April 25 at open of business.
- Variants and Genomic Surveillance for SARS-CoV-2 in Texas (last updated 4/21/22)
- ADDED: Notice of Dashboard Maintenance: The Variant Proportions in Texas dashboard is undergoing scheduled maintenance from Saturday, April 23 at 8:00 am CT through Sunday, April 24 at 5:00 pm. The dashboard will be available again Monday, April 25 at open of business.
- Information for COVID-19 Therapeutics Providers (last updated 4/18/22)
- News & Updates
- Provider Resources & Training
- Important Links
- UPDATED link: COVID-19 Therapeutics Decision Aid (ASPR)
- Important Links
- COVID-19 Therapeutics – Product Information (last updated 4/22/22)
- Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) (Pfizer)
If you have any questions or would like more information about the content on this website, please contact coronavirus@dshs.texas.gov.
DSHS Flu Surveillance Activity Report Update.
This information has recently been updated and is now available. Compared to the previous week, the percentage of specimens testing positive for influenza reported by hospital laboratories has decreased. The percentage of patient visits due to influenza-like illness (ILI) has decreased. No influenza-associated pediatric deaths were reported. No influenza-associated institutional outbreaks or school closures were reported.
DSHS RSV Data Update. This information has recently been updated and is now available. RSV surveillance in Texas is based on data collected through the National Respiratory and Enteric Virus Surveillance System ( NREVSS) sponsored by the Centers for Disease Control and Prevention (CDC). This is a laboratory-based sentinel surveillance system and participation is voluntary. Sentinel providers report both the number of RSV tests performed and the number of positive tests detected on a weekly basis. Testing is typically by antigen detection; however, viral isolation (i.e., culture) and polymerase chain reaction (PCR) testing are also performed. For the 2016-2017 season, hospital and public health laboratories submitting RSV data are located throughout the state.
Reminder: Submit Updated Training Materials. This is a reminder to Training Providers to submit updated training materials with their license renewal applications to continue teaching asbestos training courses. Review Texas Asbestos Health Protection Rules §296.71(g) for details.
Health and Human Services Commission
COVID-19 NF Webinar with CDC, HHSC LTCR, and DSHS on May 11 Nursing facility providers should attend this and all COVID-19 webinars with HHSC Long-term Care Regulation and the Texas Department of State Health Services. LTCR and DSHS will provide the latest information on the COVID-19 pandemic and take live questions from participants.
Staff from the Centers for Disease Control and Prevention will be joining the webinar as special guests to discuss nursing facility guidance related to COVID-19 infection control and contingency and crisis staffing. CDC staff will take live questions from nursing facility providers.
Provider attendance is critical to staying current with COVID-19 requirements and guidance. Internet Explorer users may have difficulties registering for the webinar. Try another browser such as Google Chrome or Microsoft Edge.
NF Provider Webinar
May 11, 2022
2:30 – 4:00 p.m.
Register for the COVID-19 webinar.
Members Sought for the Policy Council for Children and Families If you’re interested in improving services for families of children who have a disability, you may want to apply to be a member of the Policy Council for Children and Families. The Texas Health and Human Services Commission executive commissioner will appoint members to the council to serve a term expiring Dec. 31, 2025 or Dec. 31, 2026.
Applications are due by 11:59 p.m., May 15.
HHSC will consider the applicants’ qualifications, background and interest in serving on the council and will try to choose council members who represent the diversity of all Texans. For this reason, HHSC considers applicants’ ethnicity, gender and geographic location.
- Review the Policy Council for Children and Families Application for Membership application letter to find out who the council is seeking as representatives and if you qualify.
- After reviewing the application letter and verifying you meet the qualifications for at least one category, click on the application to apply to be on the council.
A council member must regularly take part in council meetings. They may also have to take part in subcommittee meetings or other related activities. Council meetings are held about once every three months in Austin or at the call of the presiding officer. To the extent permitted by the current state budget, a council member who receives services from HHSC or is a family member of a child who receives services may be repaid for their travel expenses to and from meetings if money is available and in accordance with the HHSC Travel Policy. Other council members aren’t paid to attend or travel to and from council meetings.
HHSC prefers that you submit your application electronically, but you may submit the application by email, mail or fax to the following addresses:
Email: HHS_Appointments@hhs.texas.gov Subject: PCCF
Mail: Texas Health and Human Services Commission 701 W 51st Street Mail Code 0223 Austin, TX 78751 Attn: Susanna Sparkman
Fax: 512-206-3984 Attn: PCCF
For more information about the committee visit the council website or email Viral Khakkar.
For more information about the application process, email Susanna Sparkman.
REMINDER: Medicaid Electronic Health Record Incentive Promoting Interoperability Program Audit Ad Hoc Review Panel Applications Due May 9
If you’re familiar with health information technology initiatives, specifically electronic health record systems and the Medicaid Electronic Health Record Incentive Promoting Interoperability Program, you may want to apply to be a member of the Medicaid EHR Incentive PI Program Audit Ad Hoc Review Panel. But act quickly as applications are due May 9.
Providers Encouraged to Take Part in 2022 LTC Nurse Staffing Study The Texas Center for Nursing Workforce Studies’ 2022 Long-term Care Nurse Staffing Study is open through May 31. This survey helps assess nurse staffing issues among employers of nurses throughout the state. It also gathers data helping nursing advocates and lawmakers make informed decisions about the Texas nursing shortage.
HHSC encourages directors of nursing, or other employers of nurses, to take part in the study. Your responses help ensure valid, reliable, and representative data are available to support recommendations and policy aimed at strengthening the nursing workforce in Texas. Take the survey here.
Register Now for Culture Change: Transforming Care within the Regulations In-Person Trainings This one-day interactive course was designed for all nursing facility staff to discuss culture change principles within the context of the federal rules and regulations. Type of staff include:
- Registered nurses
- Nurse’s aides
- Social workers and administrators
- Surveyors of nursing facilities
Professional staff will be introduced to:
- Strategies and practices that will enhance the quality of care provided to residents in Long-Term Care facilities.
- The qualitative and quantitative data that a facility should gather.
- Analyze before beginning a culture change initiative.
Upon successful completion this course offers:
- Six contact hours for RNs
- 7.6 contact hours for LP/VNs
- 6.5 clock hours for nursing facility administrators
- 6.5 clock hours for social workers
Join us the upcoming in-person training opportunities:
Corpus Christi
April 26
8:30 a.m. – 4:30 p.m.
Bryan/College Station
May 25
8:30 a.m. – 4:30 p.m.
Access the Joint Training registration page.
Survey: Healthcare Professionals Asked About Prescription Drug Affordability
The Health and Human Services Commission is conducting a brief survey of healthcare professionals to learn more about prescription drug affordability for Texans. The goal of the survey is to determine cost-prohibitive medications and the barriers people face in affording their medications.
The 2022-23 General Appropriations Act and House Bill (H.B.) 18, 87th Legislature, Regular Session, 2021, established the Texas Cares Program to provide uninsured individuals access to prescription drug benefits. The Health and Human Services Commission is conducting a survey of healthcare professionals to learn more about the challenges their clients/ patients face to afford their medications. This survey should take 5-10 minutes. Your response will be used to help inform the design of the program.
If you have any questions about the survey, please email us at: TexasCaresRX@hhs.texas.gov
The survey will remain open until May 4, 2022. Texas Cares Prescription Drug Affordability Survey (jotform.com)
Providers Encouraged to Take Part in 2022 Home Health and Hospice Care Nurse Staffing Study The Texas Center for Nursing Workforce Studies’ 2022 Home Health and Hospice Care Nurse Staffing Study is open through May 31.
The survey assesses nurse staffing issues among employers of nurses throughout the state. It will help gather data so nursing advocates and lawmakers can make informed decisions about the nursing shortage in Texas.
HHSC encourages directors of nursing, or other employers of nurses, to take part. This will ensure reliable and representative data are available to support recommendations and policy aimed at strengthening the nursing workforce in Texas. Take the survey here.
COVID-19 Response Plan for DAHS Providers Updated – April 19 HHSC Long-term Care Regulation has updated the COVID-19 Response Plan for DAHS Providers (PDF).
HHSC Updates the ALF COVID-19 Response Plan and FAQ Documents
HHSC Long-term Care Regulation updated the COVID-19 Response Plan and FAQ documents for assisted living facilities. Read the updated COVID-19 Response Plan for ALFs (PDF). Read the updated Frequently Asked Questions for ALFs about COVID-19 (PDF).
HHSC Updates the ICF/IID COVID-19 Response Plan and FAQ Documents
HHSC Long-term Care Regulation updated the COVID-19 Response Plan and FAQ documents for Intermediate Care Facilities for Individuals with an Intellectual Disability or Related Conditions program. HHSC Long-term Care Regulation updated the COVID-19 Response Plan and FAQ documents for Intermediate Care Facilities for Individuals with an Intellectual Disability or Related Conditions program. Read the updated ICF/IID COVID-19 Response Plan (PDF). Read the updated Frequently Asked Questions for ICF/IIDs about COVID-19 (PDF).
Office of Inspector General
No Report this week
Texas Department of Insurance
No Report This week
Texas Department of Licensing and Regulation
The adopted rules implementing Senate Bill (SB) 40, 87th Legislature. The Texas Commission of Licensing and Regulation adopted new rules at 16 Texas Administrative Code, Chapter 100, §§100.1 – 100.3, 100.11, 100.60 – 100.65, and 100.70 – 100.72, the repeal of existing rules at §§100.1, 100.10, 100.31 and 100.50, amendments to existing rules at §§100.20, 100.30, and 100.40, with the addition of subchapter titles to an existing chapter, regarding General Provisions for Health-Related Programs. The adopted rules implement Senate Bill (SB) 40, 87th Legislature, Regular Session (2021) and Tex. Occ. Code §51.501 by providing guidelines to health professionals for the use of telehealth and remote continuing education, update and amend outdated rule language, and relocate certain podiatry-specific provisions to the podiatry chapter.
The adoption justification was published in the April 22, 2022, issue of the Texas Register (47 TexReg 2128). The updated rule chapter will be made available upon its effective date of May 1, 2022.
State Auditor’s Office
No Report this week
Legislative Budget Board
No Report this week
Health Resources and Services Administration
HRSA Announces $90 Million to Support New Data-Driven Approaches for Health Centers to Identify and Reduce Health Disparities. The U.S. Department of Health and Human Services (HHS), through the Health Resources and Services Administration (HRSA), announced the availability of nearly $90 million in American Rescue Plan funding to support new data-driven efforts for HRSA Health Center Program-supported health centers and look-alikes (HRSA-designated health centers) to identify and reduce health disparities.
HRSA’s modernized data collection and reporting initiative, called Uniform Data System Patient-Level Submission (UDS+), is designed to collect more and better data on social determinants of health, while also streamlining and improving data quality reporting for health centers. This effort will enable health centers to tailor their efforts to improve health outcomes and advance health equity, more precisely targeting the needs of specific communities or patients. Read the release.
HRSA Announces $226.5 Million to Launch Community Health Worker Training Program The U.S. Department of Health and Human Services (HHS), through the Health Resources and Services Administration (HRSA), today announced the availability of $226.5 million in American Rescue Plan funding to launch the Community Health Worker Training Program. This new program will increase the number of community health workers who play a critical role in connecting people to care, including COVID care; mental health and substance use disorder prevention, treatment and recovery services; chronic disease care; and other important health services. Read the release.
HRSA Distributing $1.75 Billion in Provider Relief Fund Payments to Health Care Providers Affected by the COVID-19 Pandemic The Department of Health and Human Services (HHS), through the Health Resources and Services Administration (HRSA), today announced more than $1.75 billion in Provider Relief Fund payments to 3,680 providers across the country. With this disbursement, HRSA has distributed approximately $13.5 billion from the Provider Relief Fund to nearly 86,000 and nearly $7.5 billion in American Rescue Plan (ARP) Rural payments to more than 44,000 providers since November 2021.
“Health care providers have been tireless in protecting their communities and working to maintain access to health services during the pandemic,” said HRSA Administrator Carole Johnson. “Provider Relief Fund resources continue to make it possible for providers to recruit and retain key personnel, implement safety measures, and keep their doors open to care for their patients.” Read the release.
HRSA Awards $16 Million to Strengthen the Maternal, Infant, and Early Childhood Home Visiting Program, Announces $9 Million Available to Expand State Maternal Health Innovation and Implementation Program The Department of Health and Human Services (HHS), through the Health Resources and Services Administration (HRSA), announced approximately $16 million to strengthen Maternal, Infant, and Early Childhood Home Visiting (MIECHV) Programs through seven awards supporting eight states. These awards will advance data and technology innovations to support positive maternal and child health outcomes in states and communities, and focus on addressing health disparities. This announcement comes as the Biden-Harris Administration recognizes Black Maternal Health Week, which takes place this year from April 11 – 17, 2022. The Administration has championed policies to improve maternal health and equity and addressing the maternal mortality and morbidity crisis.
In addition, HRSA is announcing the availability of up to $9 million through the State Maternal Health Innovation and Data Capacity Program to expand the State Maternal Health Innovation and Implementation Program. This program supports state-level development and implementation of proven strategies to improve maternal health and address maternal health disparities. The new funding will continue to build state capacity to deliver high-quality maternity care services, provide training for maternal care clinicians, and enhance the quality of state-level maternal health data through better collection, reporting and analysis. The program will fund up to nine cooperative agreements, and each will receive up to $1 million over five years.
Centers for Medicare and Medicaid Services
CMS Skilled Nursing Facilities (SNF)/Long Term Care (LTC) Open Door Forum
The next CMS Skilled Nursing Facilities (SNF)/Long Term Care (LTC) Open Door Forum scheduled for:
Date: Thursday, April, 28 2022
Start Time: 2:00 PM – 3:00 PM Eastern Time (ET);
Please dial-in at least 15 minutes before call start time.
Conference Leaders: Todd Smith & Jill Darling
**This Agenda is Subject to Change**
- Opening Remarks
Chair – Todd Smith (Center for Medicare)
Moderator – Jill Darling (Office of Communications)
- Announcements & Updates
- FY 2023 SNF PPS Proposed Rule
- FY 2023 Updates to the SNF Payment Rates
- Recalibration of the PDPM Parity Adjustment
- Changes in PDPM ICD-10 Code Mappings
- Permanent Cap on Wage Index Decreases
- Request for Information: Coding Infection Isolation
- SNF Quality Reporting Program
- SNF Value-Based Purchasing Program
- PBJ Reminder
- Policy Questions should be sent to NHStaffing@cms.hhs.gov
- Technical Issues/Questions should be sent to iqies@cms.hhs.gov
- PBJ Website: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/Staffing-Data-Submission-PBJ.html
III. Open Q&A
**DATE IS SUBJECT TO CHANGE**
Next ODF: TBD
Mailbox: SNF_LTCODF-L@cms.hhs.gov
Nursing Facility Open Door Forum. This Open Door Forum is open to everyone, but if you are a member of the Press, you may listen in but please refrain from asking questions during the Q & A portion of the call. If you have inquiries, please contact CMS at Press@cms.hhs.gov. Thank you.
Open Door Participation Instructions:
This call will be Conference Call Only.
To participate by phone:
Dial: 1-888-455-1397 & Conference Passcode: 5109694
Persons participating by phone do not need to RSVP. TTY Communications Relay Services are available for the Hearing Impaired. For TTY services dial 7-1-1 or 1-800-855-2880. A Relay Communications Assistant will help.
Instant Replay: 1-866-462-8997; Conference Passcode: No Passcode Needed
Instant Replay is an audio recording of this call that can be accessed by dialing 1-866-462-8997 Instant Replay begins 1 hour after the call has ended. The recording expires April 30, 2022, 11:59 PM ET
For ODF schedule updates and E-Mailing List registration, visit our website at http://www.cms.gov/OpenDoorForums/.
We encourage you to visit our CMS Podcasts and Transcript webpage where you can listen and view the most recent Skilled Nursing Facilities (SNF)/Long Term Care (LTC) ODF call at https://www.cms.gov/Outreach-and-Education/Outreach/OpenDoorForums/PodcastAndTranscripts.html. Thank you.
CMS provides free auxiliary aids and services including information in accessible formats. Click here for more information. This will point partners to our CMS.gov version of the “Accessibility & Nondiscrimination notice” page.
CMS Reweighting 2021 MIPS Cost Performance CategoryThe Centers for Medicare & Medicaid Services (CMS) recognizes the impact that the COVID-19 pandemic public health emergency (PHE) continued to have on clinicians and the services they provided in the 2021 performance period.
Due to COVID-19’s impact on cost measures, we’re reweighting the cost performance category from 20% to 0% for the 2021 performance period. The 20% cost performance category weight will be redistributed to other performance categories in accordance with § 414.1380(c)(2)(ii)(E). Please see the table below for reweighting scenarios.
Why CMS is Reweighting the MIPS Cost Performance Category for 2021
Cost was already reweighted to 0% for all individual MIPS eligible clinicians, even if data were submitted for other performance categories, due to the automatic extreme and uncontrollable circumstances (EUC) policies under § 414.1380(c)(2)(i)(A)(6) and § 414.1380(c)(2)(i)(C). Our analysis of the underlying data for the 2021 performance period shows similar results at the group- and individual-level across measures. As a result, we believe that reweighting shouldn’t depend on whether you choose to report as a group or individual.
Given these circumstances and in accordance with § 414.1380(c)(2), we’ll assign a weight of 0% to the cost performance category for the 2021 performance period and redistribute the prescribed weight of 20% to another performance category or categories.
Specifically, we don’t believe we can reliably calculate scores for some of the cost measures that would adequately capture and reflect the performance of MIPS eligible clinicians based on the following reasons, as shown by our analysis of the cost performance category data for the 2021 performance period:
- Most measures have higher observed and risk-adjusted costs at the episode-level. This indicates that risk adjustment at the episode-level doesn’t entirely account for differences in resource use, particularly for broader measures or measures that are clinically proximate to respiratory disease and COVID-19.
- There’s less of an effect at the provider-level for most measures where testing shows that scores don’t appear to be adversely impacted by higher case-loads of episodes with a recent or concurrent COVID-19 diagnosis. However, there are a small number of measures where scores may be adversely affected by the volume of episodes with a COVID-19 diagnosis.
Please note that starting with the 2022 performance period, instead of reweighting the entire cost performance category, individual cost measures can be suppressed if the data used to calculate the score was impacted by significant changes during the performance period, such that calculating the cost measure would lead to misleading or inaccurate results. This provision allowing greater flexibility was finalized in the CY 2022 Physician Fee Schedule Final Rule.
Clinicians don’t need to take any action as a result of this decision because the cost performance category relies on administrative claims data.
MIPS Performance Category Weight Redistribution Policies Finalized for the 2021 Performance Period
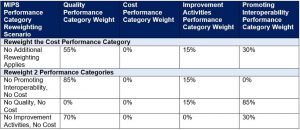
The table below illustrates the MIPS performance category weights and reweighting policies that apply to MIPS eligible clinicians, groups and virtual groups in the 2021 performance period.
*This table can be found at § 414.1380(c)(2)(ii)(E).
This reweighting of the cost performance category applies in addition to the extreme and uncontrollable circumstances (EUC) policies under § 414.1380(c)(2)(i)(A)(6) and § 414.1380(c)(2)(i)(C).
- Cost was already reweighted to 0% for all individual MIPS eligible clinicians, even if data were submitted for other performance categories, due to the automatic EUC policy.
- Cost will now be reweighted to 0% for all groups and virtual groups, even if they didn’t request reweighting through an EUC exception application.
As a reminder, under § 414.1380(c), if a MIPS eligible clinician is scored on fewer than 2 performance categories (meaning 1 performance category is weighted at 100% or all performance categories are weighted at 0%), they’ll receive a final score equal to the performance threshold and a neutral MIPS payment adjustment for the 2023 MIPS payment year.
Cost Data in Performance Feedback for 2021
We recognize that this is the second year that we’ve had to reweight the cost performance category due to COVID-19, and that clinicians need more insight into and familiarity with their performance in this category. To support this need, we’ll provide patient-level reports on the 2021 cost measures for which clinicians, groups and virtual groups met the case minimum. Patient-level reports will be available as part of the final performance feedback in August 2022.
Please note that we won’t include measure-level scoring information in performance feedback. As previously mentioned, we don’t believe we can reliably calculate scores for the cost measures that would adequately capture and reflect the performance of MIPS eligible clinicians.
Questions?
Please contact the Quality Payment Program at 1-866-288-8292 or by e-mail at: QPP@cms.hhs.gov. To receive assistance more quickly, consider calling during non-peak hours – before 10 a.m. and after 2 p.m. ET.
- Customers who are hearing impaired can dial 711 to be connected to a TRS Communications Assistant.
The Inpatient Rehabilitation Facility (IRF) and Long-Term Care Hospital (LTCH) Updated Guidance Virtual Training Program
REGISTRATION OPEN: The Inpatient Rehabilitation Facility (IRF) and Long-Term Care Hospital (LTCH) Updated Guidance Virtual Training Program
The Centers for Medicare & Medicaid Services (CMS) will be providing a virtual training program that will review the updated guidance for the Inpatient Rehabilitation Facility – Patient Assessment Instrument (IRF-PAI) 4.0 and the LTCH Continuity Assessment Record and Evaluation (CARE) Data Set (LCDS) 5.0 for providers in the IRF and LTCH settings. This training is part of a comprehensive strategy to ensure IRF and LTCH providers have access to the education necessary to understand and comply with changes in reporting requirements associated with the IRF and LTCH Quality Reporting Programs (QRPs) that go into effect on October 1, 2022. A major focus of this training will be on the cross-setting implementation of the standardized patient assessment data elements being introduced in 2022 to ensure more consistent reporting and evaluation across post-acute care settings.
The training program consists of two parts:
- Part 1: Beginning May 9, 2022: Access will be provided to recorded training session videos that deliver foundational knowledge necessary to understand the new items and guidance. These videos are intended to be reviewed in advance of the live event.
- Part 2: June 15–16, 2022: Live, virtual workshop sessions will provide coding practice on the items covered in the Part 1 videos. These live sessions will take place each day between 1 p.m. and 5 p.m. ET.
Registration can be completed online through the following link: https://www.regpack.com/reg/IRF-LTCH
If you have questions about accessing resources or feedback regarding the trainings, please email the PAC Training Mailbox. Content-related questions should be submitted to the LTCH QRP Help Desk and IRF QRP Help Desk
Register Today for the CMS Framework for Health Equity Symposium
On Thursday, April 28, 2022, at 1:00 p.m. ET, the Centers for Medicare & Medicaid Services Office of Minority Health (CMS OMH) will host a CMS Framework for Health Equity Symposium to discuss the CMS Framework for Health Equity 2022-2032 a comprehensive 10-year approach to further embed health equity across all CMS programs including Medicare, Medicaid, the Children’s Health Insurance Program, and the Health Insurance Marketplaces.
During the symposium, CMS OMH will cover HHS and CMS equity plans initiatives and the CMS Framework for Health Equity, including the importance of data collection, the connection to stakeholder and partner efforts to improve health equity, and additional health equity information and updates from CMS.
Symposium Details
Title: CMS Framework for Health Equity Symposium
Date: Thursday, April 28
Time: 1:00 p.m. – 2:30 p.m. ET
To Register: https://us06web.zoom.us/webinar/register/WN_RwkwJfq4TECoetIeCSS3ew
We will post additional information concerning the webinar on our Webinar & Events webpage.
Please note: You will receive an email with login information from Zoom upon registration. If you do not receive an email in a few days’ time, please contact CMSOMHTeam@ketchum.com. Space is limited; we encourage early registration to ensure you can attend.
To learn more about the CMS Office of Minority Health, visit https://go.cms.gov/omh or contact OMH@cms.hhs.gov.
Announcing the CMS Framework for Health Equity
The CMS Office of Minority Health (CMS OMH) has released the CMS Framework for Health Equity. This Framework challenges us to incorporate health equity and efforts to address health disparities as a foundational element across all our work — in every program and across every community. Using five priority areas, CMS will use this framework to design, implement, and operationalize policies and programs to support health for all people served by our programs, eliminating avoidable differences in health outcomes experienced by people who are disadvantaged or underserved, and providing the care and support that our enrollees need to thrive.

Across our Centers and Offices, we are committed to taking an integrated, action-oriented approach to identify and remedy systemic barriers to equity so that every one of the people we serve has a fair and just opportunity to attain their optimal health regardless of race, ethnicity, disability, sexual orientation, gender identity, socioeconomic status, geography, preferred language, or other factors that affect access to care and health outcomes.
The CMS Framework for Health Equity identified high-impact priorities based on stakeholder engagement, a review of the evidence base, and discussions across federal partners. It updates the previous Medicare-focused CMS Equity Plan for Improving Quality in Medicare with an enhanced and more comprehensive 10-year approach to further embed health equity across Medicare, Medicaid, CHIP, and the Health Insurance Marketplaces. The updated CMS Framework for Health Equity also brings focus to CMS’ work supporting health care organizations, health care professionals and partners—providers, health plans, federal, state, and local partners, tribal nations, individuals and families, quality improvement partners, researchers, policymakers, and other stakeholders—in activities to achieve health equity.
Thursday, April 28, 2022, 1:00pm-2:30pm ETJoin us to learn more! CMS OMH will host a virtual symposium to showcase the CMS Framework for Health Equity and detail the 5 priority areas. Join us to learn more:
Hospice Visits in the Last Days of Life On April 14, 2022, CMS inadvertently posted an announcement about the National Quality Forum (NQF) endorsement of the claims-based measure, Hospice Visits in the Last Days of Life, or HVLDL as NQF #3645. CMS removed the announcement and will provide an update as appropriate.
Reminder: Register for CMS Web Interface and CAHPS for MIPS Survey for 2022 Performance Period Registration is open for the CMS Web Interface and Consumer Assessment of Healthcare Providers and Systems (CAHPS) for the Merit-based Incentive Payment System (MIPS) Survey for the 2022 performance period.
- Groups, virtual groups, and Alternative Payment Model (APM) Entities with 25 or more clinicians (including at least one MIPS eligible clinician) can register through June 30, 2022, to use the CMS Web Interface for reporting quality measures under traditional MIPS.
- Groups, virtual groups, and APM Entities with 2 or more clinicians (including at least one MIPS eligible clinician) can register through 2022 to administer the CAHPS for MIPS Survey under traditional MIPS or the Alternative Payment Model (APM) Performance Pathway (APP).
- Groups, virtual groups and APM Entities only need to register if they intend to report through the CMS Web Interface and/or administer the CAHPS for MIPS Survey for the 2022 performance period.
NOTE: Medicare Shared Savings Program Accountable Care Organizations (Shared Savings Program ACOs) don’t need to register. Shared Savings Program ACOs are automatically registered for the CMS Web Interface and CAHPS for MIPS Survey, as they’re required to meet reporting requirements for the quality performance category under the APM Performance Pathway (APP).
- While Shared Savings Program ACOs are automatically registered for the CMS Web Interface, they aren’t required to report their quality data via the CMS Web Interface. Alternatively, they can choose to meet the APP quality requirements by reporting the 3 required electronic clinical quality measures (eCQMs) or MIPS clinical quality measures (MIPS CQMs).
- The CAHPS for MIPS Survey is required under the APP. Though automatically registered, Shared Savings Program ACOs will still need to hire a CMS-approved vendor to administer the CAHPS for MIPS Survey.
How to Register
If your group, virtual group, or APM Entity (other than a Shared Savings Program ACO) would like to submit quality measures for traditional MIPS using the CMS Web Interface and/or administer the CAHPS for MIPS Survey (for traditional MIPS or the APP) for the 2022 performance period, you must register by 8 p.m. ET on June 30, 2022. You may edit or cancel your registration at any time during the registration period.
To register, please log in to the Quality Payment Program (QPP) website. You’ll need to have the Security Official role in order to register your organization. Please refer to the QPP Access User Guide (ZIP) for information about obtaining a Security Official role for your organization. You can register by:
- Signing in to QPP.
- Going to the Manage Access page.
- Clicking “Edit Registration” by 8 p.m. ET on June 30, 2022.
Please note the following:
- Groups, virtual groups, and APM Entities that submitted data for the quality performance category via the CMS Web Interface for the 2021 performance period will be automatically registered for the CMS Web Interface for the 2022 performance period, unless you cancel your registration.
- However, if your group, virtual group, or APM Entity registered to administer the CAHPS for MIPS Survey for the 2021 performance period, you’ll need to register to administer the CAHPS for MIPS Survey for the 2022 performance period.
- Registered groups, virtual groups, and APM Entities (including Shared Savings Program ACOs) that elect to administer the CAHPS for MIPS Survey will need to hire a CMS-approved vendor to administer the survey. If you don’t plan to administer the CAHPS for MIPS Survey for the 2022 performance period, we encourage you to cancel your registration by 8 p.m. ET on June 30, 2022.
Medicare Provider Compliance News
- Hospice Quality Reporting Program: Key Dates & Measure Change
- Ambulance Ground Transport: Comparative Billing Report in April
- Hospices: Aggregate & Inpatient Caps under the Value-Based Insurance Design Model
2020 Medicare Current Beneficiary Survey (MCBS) Early Look Data Brief
CMS is pleased to release the 2020 Medicare Current Beneficiary Survey (MCBS) Early Look Data Brief. Using preliminary data for community-dwelling Medicare beneficiaries from the 2020 MCBS Survey File, the Early Look presents selected measures that are not available in the CMS administrative data, including beneficiary health status and functioning, access to care, satisfaction with care, and primary language spoken at home. The Early Look is being released in advance of the upcoming MCBS 2020 Survey File LDS release anticipated in summer 2022.
You may access the full data brief here.
CMS Outlines Strategy to Advance Health Equity, Challenges Industry Leaders to Address Systemic Inequities
CMS Administrator issues invitation to health care industry to advance health equity.
Today, the Centers for Medicare & Medicaid Services (CMS) outlined an action plan that demonstrates the Biden-Harris Administration’s ongoing efforts to provide high-quality, affordable health care for all people, regardless of their background, and to drive health equity across the Department of Health and Human Services (HHS). Building on the agency’s commitment to make health equity the first “pillar” of its strategic vision, CMS Administrator Chiquita Brooks-LaSure invited health care industry leaders to make commitments to advance health equity and work with CMS to share best practices to address systemic inequities in the delivery of care.
“Advancing health equity is the core work of the Centers for Medicare & Medicaid Services. We can’t achieve our health system goals until everyone can attain the highest level of health. That’s why I am inviting the health care industry to work alongside CMS as we transform the way patients are cared for in our country,” said CMS Administrator Chiquita Brooks-LaSure. “Health equity will be embedded within the DNA of CMS and serve as the lens through which we view all of our work. Our vision is clear and our goal is straightforward — we will not stop until every person has a fair and just opportunity to attain their optimal health.”
As a part of its plan, CMS laid out the central role health equity will play in the work of all CMS Centers and Offices, including the Center for Medicare (CM), the Center for Medicaid & CHIP (CMCS), Center for Consumer Information and Insurance Oversight (CCIIO), the Center for Medicare and Medicaid Innovation (CMMI), and the Center for Clinical Standards and Quality (CCSQ). This work includes working with and sharing best practices across states, health care facilities, providers, insurance companies, pharmaceutical companies, people with lived experience, researchers, and other key stakeholders to drive commitments to advance health equity.
CMS Health Equity Strategy:
CMS Administrator Chiquita Brooks-LaSure has charged each CMS Center and Office with building health equity into their core work, aimed to better identify and respond to inequities in health outcomes, barriers to coverage, and access to care. They include the following actions:
- Close gaps in health care access, quality, and outcomes for underserved populations.
- Promote culturally and linguistically appropriate services to ensure understandable and respectful care and services that are responsive to preferred languages, health literacy, and other diverse communication needs.
- Build on outreach efforts to enroll eligible people across Medicare, Medicaid/CHIP and the Marketplace.
- Expand and standardize the collection and use of data, including on race, ethnicity, preferred language, sexual orientation, gender identity, disability, income, geography, and other factors across CMS programs.
- Evaluate policies to determine how CMS can support safety net providers caring for underserved communities, and ensure care is accessible to those who need it.
- Ensure engagement with and accountability to the communities served by CMS in policy development and the implementation of CMS programs.
- Incorporate screening for and promote broader access to health-related social needs, including greater adoption of related quality measures, coordination with community-based organizations, and collection of social needs data in standardized formats across CMS programs and activities.
- Ensure CMS programs serve as a model and catalyst to advance health equity through our nation’s health care system, including with states, providers, plans, and other stakeholders.
- Promote the highest quality outcomes and safest care for all people through use of the framework under the CMS National Quality Strategy.
Invitation to Advance Health Equity
In effort to address systemic inequities across the industry, CMS will be encouraging health care leaders to make commitments to advance health equity, such as designing, implementing, and operationalizing initiatives that support health; eliminating avoidable differences in health outcomes experienced by people who are disadvantaged or underserved; and providing the care and support people, particularly those with Medicare, Medicaid or Marketplace coverage, need to thrive. This aligns with the Biden-Harris Administration’s comprehensive, long-term strategy to advance health equity.
To ensure sustained progress through meaningful initiatives, CMS will convene industry stakeholders, including health care facilities, insurance companies, state officials and providers. The first of these convenings will take place in Summer 2022 and focus on ways to improve maternal health outcomes experienced by pregnant and postpartum people. CMS and experts will invite health care industry leaders to share best practices and commitments to strengthen maternal health.
For more information, please visit: www.cms.gov/sites/default/files/2022-04/Health%20Equity%20Pillar%20Fact%20Sheet_1.pdf
HHS Releases New Data and Report on Hospital and Nursing Home Ownership
New data supports President Biden’s plan to protect seniors by improving safety and quality of care
Today, the Department of Health and Human Services (HHS) is taking actions to promote competition and transparency in our nation’s health care system that can improve the safety and quality of nursing homes and hospitals. The Centers for Medicare & Medicaid Services (CMS) is releasing data publicly — for the first time — on mergers, acquisitions, consolidations, and changes of ownership from 2016-2022 for hospitals and nursing homes enrolled in Medicare. This data is a powerful new tool for researchers, state and federal enforcement agencies, and the public to better understand the impacts of consolidation on health care prices and quality of care.
HHS’s Office of the Assistant Secretary for Planning and Evaluation (ASPE) is also releasing a related report — an analysis of the new CMS data examining trends in changes of ownership over the past six years.
These new data and analysis support President Joe Biden’s Executive Order on Promoting Competition, and advance the Biden-Harris Administration’s goal of improving transparency around nursing facility ownership and enhancing nursing home safety and quality, as outlined in President Biden’s State of the Union Action Plan for Protecting Seniors by Improving Safety and Quality of Care in the Nation’s Nursing Homes.
“Today, for the first time, we are releasing data on the impact of hospital and nursing home consolidation for people across our nation. By improving the quality of reporting by hospitals and nursing homes on ownership and consolidation, we also advance President Biden’s agenda to promote competition, lower health care costs for American families, and protect patients,” said HHS Secretary Xavier Becerra.
“Hospital and nursing facility consolidation leaves many underserved areas with inadequate or more expensive health care options,” said CMS Administrator Chiquita Brooks-LaSure. “This new data gives researchers, state and federal enforcement agencies, and the public new opportunities to examine how mergers, acquisitions, consolidations, and changes of ownership impact access to care, care quality, and prices as a way to enable greater transparency and insight into the hospital and nursing home industries.”
CMS’s data on the changes of ownership — which includes details on mergers, acquisitions, and consolidations — is now available on data.cms.gov. The data can help researchers, enforcers, and the public analyze trends and issues in health care markets, and more specifically, provide insight into how the ownership of health care providers impacts costs and outcomes of consumers. For example, ASPE’s report identifies several findings from the new dataset:
- Changes of ownership have been much more common in nursing homes than hospitals over the past six years.
- There is also wide ownership variation by state. For instance, 19% of hospitals (14 out of 73) in South Carolina were sold during this period, while most states had fewer than 4% of hospitals change ownership.
- A majority (62.3%) of Skilled Nursing Facilities (SNFs) that were purchased have a single organizational owner, 6.9% have multiple organizations owners, while 18.2% have only individual owners and 12.7% have both types of owners.
CMS expects to release updated change of ownership data on a quarterly basis. The CMS data will enhance transparency for hospitals and nursing homes patients, potential patients and their loved ones, as well as for policymakers and the communities where these facilities are located.
This new data release is just one of many steps HHS is taking to increase transparency and accountability, and to boost competition in the health care industry.
For more information on the HHS data release, including publicly available data files, please visit: Hospital Change of Ownership: https://data.cms.gov/provider-characteristics/hospitals-and-other-facilities/hospital-change-of-ownership; and Skilled Nursing Facility Change of Ownership: https://data.cms.gov/provider-characteristics/hospitals-and-other-facilities/skilled-nursing-facility-change-of-ownership.
For the ASPE report analyzing the new change in ownership data, please visit: https://aspe.hhs.gov/reports/changes-ownership-hospital-skilled-nursing-facilities
CMS Proposes Policies to Advance Health Equity and Maternal Health, Support Hospitals Today, the Centers for Medicare & Medicaid Services (CMS) issued a proposed rule for inpatient and long-term hospitals that builds on the Biden-Harris Administration’s key priorities to advance health equity and improve maternal health outcomes. In addition to annual policies that promote Medicare payment accuracy and hospital stability, the FY 2023 Inpatient Prospective Payment System (IPPS) and Long-Term Care Hospital (LTCH)
Prospective Payment System (PPS) rule includes measures that will encourage hospitals to build health equity into their core functions, thereby improving care for people and communities who are disadvantaged and/or underserved by the healthcare system. The rule includes three health equity-focused measures in hospital quality programs, seeks stakeholder input related to documenting social determinants of health in inpatient claims data, and proposes a “Birthing-Friendly” hospital designation.
For acute care hospitals paid under the IPPS that successfully participate in the Hospital Inpatient Quality Reporting Program and are meaningful electronic health record users, the proposed increase in operating payment rates is projected to be 3.2%. This reflects a FY 2023 projected hospital market basket update of 3.1% reduced by a projected 0.4 percentage point productivity adjustment and increased by a 0.5 percentage point adjustment required by statute. Under the LTCH PPS, CMS expects payments to increase by approximately 0.8% or $25 million.
“Building a healthier America starts with ensuring everyone in our nation has access to high-quality, affordable health care,” said HHS Secretary Xavier Becerra. “The new Medicare policies we are proposing today will help advance health equity in our health systems and dramatically improve maternal care for new parents and their newborns.”
“This rule, which funds a substantial portion of Medicare programs, is crucial to the foundation of CMS’ vision, ensuring access for all people with Medicare and maintaining incentives for our hospital partners to operate efficiently,” said CMS Administrator Chiquita Brooks-LaSure. “This year—through a health equity lens—we are also re-envisioning the next chapter of health care quality and patient safety.”
Advancing Health Equity
Health equity means the attainment of the highest level of health for all people, where everyone has a fair and just opportunity to attain their optimal health regardless of race, ethnicity, disability, sexual orientation, gender identity, socioeconomic status, geography, preferred language, or other factors that affect access to care and health outcomes. CMS is working to advance health equity by designing, implementing, and operationalizing policies and programs that support health for all the people served by our programs, eliminating avoidable differences in health outcomes experienced by people who are disadvantaged or underserved, and providing the care and support that our enrollees need to thrive.
To address health care disparities in hospital inpatient care and beyond, CMS is proposing three health equity-focused measures for adoption in the Hospital Inpatient Quality Reporting (IQR) Program. The first measure assesses a hospital’s commitment to establishing a culture of equity and delivering more equitable health care by capturing concrete activities across five key domains, including strategic planning, data collection, data analysis, quality improvement, and leadership engagement. The second and third measures capture screening and identification of patient-level, health-related social needs—such as food insecurity, housing instability, transportation needs, utility difficulties, and interpersonal safety. By screening for and identifying such unmet needs, hospitals will be in a better position to serve patients holistically by addressing and monitoring what are often key contributors to poor physical and mental health outcomes.
Additionally, CMS seeks public input on how to optimally measure health care quality disparities, including what to prioritize in data collection and reporting as well as approaches to consider in driving provider accountability across hospital quality programs.
CMS is also proposing to discontinue the use of proxy data for uncompensated care costs in determining uncompensated care payments for Indian Health Service and Tribal hospitals and hospitals in Puerto Rico, and to establish a new supplemental payment to prevent undue long-term financial disruption for these hospitals.
Improving Care for People Experiencing Homelessness and Documenting Social Determinants of Health
CMS is seeking stakeholder input through a Request for Information (RFI) on social determinants of health, particularly related to homelessness, reported by hospitals on Medicare claims. Consistently documenting these factors could better support people experiencing homelessness and more fully consider resources expended by hospitals. With this RFI, CMS seeks to better understand the perspectives of people who are experiencing or have experienced homelessness, advocates representing people experiencing homelessness, hospitals and other key stakeholders for consideration in future payment policies.
Improving Maternal Health Outcomes
CMS is proposing the creation of a new hospital designation to identify “birthing friendly hospitals” and additional quality measure reporting to drive improvements in maternal health outcomes and maternal health equity.
The Biden-Harris Administration has championed policies to improve maternal health and equity since President Joe Biden and Vice President Kamala Harris first took office. This week, Vice President Harris convened a first-ever White House meeting with Cabinet Secretaries and agency leaders, including Secretary Becerra, CMS Administrator Chiquita Brooks-LaSure, and Health Resources and Services Administration (HRSA) Administrator Carole Johnson, to discuss the Administration’s whole-of-government approach to reducing maternal mortality and morbidity. In December 2021, Vice President Harris announced a historic Call to Action to improve health outcomes for parents and their young children in the United States. Today’s announcement is part of the Biden-Harris Administration’s continued response to that Call to Action.
“Improving maternal health outcomes—particularly among underserved communities and groups that we know experience adverse birth outcomes at a higher rate—is a top priority for the Biden-Harris Administration, CMS, and for me personally,” said Administrator Brooks-LaSure.
The “Birthing-Friendly” hospital designation would assist consumers in choosing hospitals that have demonstrated a commitment to maternal health through implementation of best practices that advance health care quality, safety, and equity for pregnant and postpartum patients. Initially, the designation would be awarded to hospitals based on their attestation to the Hospital IQR Program’s Maternal Morbidity Structural Measure.
The Maternal Morbidity Structural Measure reflects hospitals’ commitment to the quality and safety of maternity care they furnish. Data will be submitted by hospitals for the first time in May 2022, and CMS will post data for October to December 2021 in fall 2022. The hospital designation would begin in fall 2023. Criteria for the designation could be expanded in the future.
The proposed rule also introduces two additional quality measures for the Hospital IQR Program intended to drive improvements in maternal health, including a measure of low-risk Cesarean deliveries and a measure of severe obstetric complications.
Promoting Payment Accuracy and Stability
At its core, the rule drives high-quality, person-centered care and promotes fiscal stewardship of the Medicare program by proposing updates to Medicare fee-for-service payment rates and policies for acute care inpatient hospitals and long-term care hospitals for FY 2023. Annually, IPPS and LTCH spending accounts for more than 25% of fee-for-service Medicare spending for approximately 3,900 inpatient and long-term care hospitals.
Additional items in the proposed rule related to payment stability for hospitals, include a policy that smooths out significant year-to-year changes in hospitals’ wage indexes and a solicitation for comments on payment adjustments for purchasing domestically made surgical N95 respirators. Specifically, CMS is proposing to apply a 5% cap on any decrease to a hospital’s wage index from its wage index in the prior fiscal year; and is considering the appropriateness of payment adjustments accounting for additional costs of purchasing surgical N95 respirators made in the U.S.
For a fact sheet on the proposed payment rule visit: https://www.cms.gov/newsroom/fact-sheets/fy-2023-hospital-inpatient-prospective-payment-system-ipps-and-long-term-care-hospitals-ltch-pps
For a fact sheet specific to the maternal health and health equity measures included in the proposed payment rule visit: https://www.cms.gov/newsroom/fact-sheets/fy-2023-hospital-inpatient-prospective-payment-system-ipps-and-long-term-care-hospitals-ltch-pps-0
The White House statement on Reducing Maternal Mortality and Morbidity, as part of the first-ever federal maternal health day of action, can be viewed at: https://www.whitehouse.gov/briefing-room/statements-releases/2021/12/07/fact-sheet-vice-president-kamala-harris-announces-call-to-action-to-reduce-maternal-mortality-and-morbidity/
For a fact sheet on additional steps to address maternal health announced as part of the first-ever meeting with cabinet officials on maternal health hosted by Vice President Harris visit: https://www.whitehouse.gov/briefing-room/statements-releases/2022/04/13/fact-sheet-biden-harris-administration-announces-additional-actions-in-response-to-vice-president-harriss-call-to-action-on-maternal-health/
The FY 2023 IPPS/LTCH PPS proposed rule has a 60-day comment period. The proposed rule can be downloaded from the Federal Register at: https://www.federalregister.gov/public-inspection/current
Congressional Budget Office
No Report This week
US Food and Drug Administration
No Report This week
National Institutes of Health
Vision improvement is long-lasting with treatment for blinding blood vessel condition
NIH-funded study finds many patients with retinal vein occlusion have vision benefits, but require long-term monitoring and treatment.
New research shows that a treatment for retinal vein occlusion yields long-lasting vision gains, with visual acuity remaining significantly above baseline at five years. However, many patients require ongoing treatment. Retinal vein occlusion is one of the most common blinding conditions in the United States; without treatment, central retinal vein occlusion (CRVO), the most severe type of retinal vein occlusion often leads to significant and permanent vision loss. A report on five-year outcomes of the Study of Comparative Treatments for Retinal Vein Occlusion 2 (SCORE2), was published April 21 in American Journal of Ophthalmology. SCORE2 was funded in part by the National Eye Institute (NEI), a part of the National Institutes of Health.
Retinal vein occlusion is caused by a blockage of the veins carrying blood away from the retina, the light-sensitive tissue at the back of the eye. This blockage can lead to macular edema where fluid becomes trapped within and under the retina, leading to rapid and severe loss of visual acuity. Without treatment, this condition typically leads to permanent loss of vision. The most effective treatment, injections of anti-vascular endothelial growth factor (VEGF) drugs, helps control blood vessel leakage and swelling in the retina.
“While anti-VEGF therapy is associated with significant improvement in both retinal swelling and visual acuity in patients with central or hemi-retinal vein occlusion, our findings show that most of the patients followed still required treatment to control the macular edema for at least five years,” said Ingrid U. Scott, M.D., M.P.H., Penn State College of Medicine, Hershey, chair of the study. “This demonstrates the importance of continued monitoring of these patients.”
In 2017, SCORE2 clinical trial investigators reported that two types of anti-VEGF treatment were equally effective at improving visual acuity in people with macular edema due to CRVO or hemi-retinal vein occlusion (HRVO). CRVO affects the entire retina, while HRVO generally affects about half of the retina. Half of the study participants had been given Avastin (bevacizumab) while the other half received Eylea (aflibercept). Both drugs were administered by injection once per month for six months. At the six-month mark, the vision of participants in both groups had, on average, improved over three lines on an eye chart.
As detailed in this new report, the study investigators followed SCORE2 participants for five years, collecting information about their visual acuity, treatments, and whether their macular edema had resolved. After the initial 12-month study period, participants were treated at their physician’s discretion. Most physicians reduced the frequency of anti-VEGF injections and some switched their patients to the other anti-VEGF drug. At five years, many participants had lost some visual acuity when compared to their acuity at the 12-month mark; however, they retained on average three lines of improvement, compared to their acuity at the beginning of the study.
“It was surprising to us that despite many participants still needing treatment after five years, their visual acuity outcome remained very good,” said Michael Ip, M.D., co-chair of the study from Doheny Eye Institute, University of California Los Angeles. “In comparison to this treatment for wet age-related macular degeneration, where initial vision improvements fade over time, these results are quite favorable.”
“This five-year study tells us a lot about what’s happening with retinal vein occlusion patients in the real world,” said Scott. “Prior to this study, retinal vein occlusion was widely considered an acute illness. This study shows that RVO is a chronic disease. It also underscores the importance of disease monitoring and individualized treatment to achieve the best possible vision.”
Asthma, allergy risk may be higher for children conceived with infertility treatment Children conceived with infertility treatment may have a higher risk for asthma and allergies, suggests a study by researchers at the National Institutes of Health. The study was conducted by scientists at the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of Environmental Health Sciences, part of the National Institutes of Health. It appears in Human Reproduction.
The study enrolled approximately 5,000 mothers and 6,000 children born between 2008 and 2010. Mothers responded periodically to questionnaires on their health and their children’s health and medical histories. Infertility treatments included in vitro fertilization (sperm and egg are combined in a laboratory dish and inserted in the uterus), drugs that stimulate ovulation, and a procedure in which sperm are inserted into the uterus.
Compared to children conceived without infertility treatment, children conceived after treatment were more likely to have persistent wheeze by age 3, a potential indication of asthma. At 7 to 9 years old, children conceived with treatment were 30% more likely to have asthma, 77% more likely to have eczema (an allergic condition resulting in rashes and itchy skin) and 45% more likely to have a prescription for an allergy medication.
The authors called for additional research to determine how infertility treatment or lower parental fertility might influence the development of asthma and allergy in children.

